Complete The Balanced Neutralization Equation For The Reaction Below
Holbox
Mar 16, 2025 · 6 min read
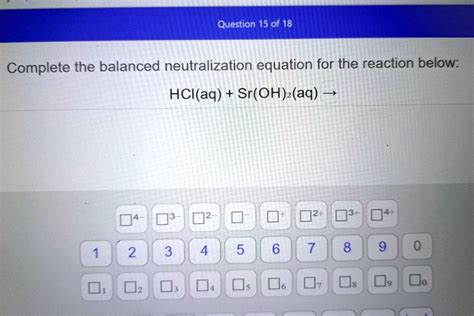
Table of Contents
- Complete The Balanced Neutralization Equation For The Reaction Below
- Table of Contents
- Complete Balanced Neutralization Equations: A Deep Dive into Acid-Base Reactions
- Understanding Neutralization Reactions
- Types of Acids and Bases
- Acids:
- Bases:
- Balancing Neutralization Equations: A Step-by-Step Approach
- Examples of Balanced Neutralization Equations
- Applications of Neutralization Reactions
- Advanced Considerations
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Complete Balanced Neutralization Equations: A Deep Dive into Acid-Base Reactions
Neutralization reactions are fundamental concepts in chemistry, representing the reaction between an acid and a base to produce a salt and water. Understanding how to balance these equations is crucial for various applications, from predicting reaction outcomes to calculating stoichiometric quantities in titrations. This comprehensive guide delves into the intricacies of neutralization reactions, providing a step-by-step approach to balancing equations and exploring different types of acids and bases involved.
Understanding Neutralization Reactions
A neutralization reaction is essentially a double displacement reaction where the acid's hydrogen ions (H⁺) combine with the base's hydroxide ions (OH⁻) to form water (H₂O). The remaining ions from the acid and base then form a salt. The overall reaction is often exothermic, releasing heat.
Key Characteristics:
- Acid + Base → Salt + Water This is the general form of a neutralization reaction.
- pH Change: The reaction results in a decrease in the pH of a basic solution and an increase in the pH of an acidic solution, moving the solution closer to a neutral pH of 7.
- Heat Release: Neutralization reactions are typically exothermic, meaning they release heat into the surroundings. This heat release can be measured to determine the enthalpy change of the reaction.
- Salt Formation: The salt produced is an ionic compound formed from the cation of the base and the anion of the acid. The properties of this salt can vary significantly depending on the specific acid and base involved.
Types of Acids and Bases
Before delving into balancing neutralization equations, it's crucial to understand the different types of acids and bases:
Acids:
- Strong Acids: These acids completely dissociate in water, releasing all their hydrogen ions. Examples include hydrochloric acid (HCl), sulfuric acid (H₂SO₄), and nitric acid (HNO₃).
- Weak Acids: These acids partially dissociate in water, releasing only a small fraction of their hydrogen ions. Examples include acetic acid (CH₃COOH), carbonic acid (H₂CO₃), and hydrofluoric acid (HF).
Bases:
- Strong Bases: These bases completely dissociate in water, releasing all their hydroxide ions. Examples include sodium hydroxide (NaOH), potassium hydroxide (KOH), and calcium hydroxide (Ca(OH)₂).
- Weak Bases: These bases partially dissociate in water, releasing only a small fraction of their hydroxide ions. Examples include ammonia (NH₃) and methylamine (CH₃NH₂).
Balancing Neutralization Equations: A Step-by-Step Approach
Balancing a neutralization equation ensures that the number of atoms of each element is the same on both the reactant and product sides of the equation. Here's a step-by-step approach:
1. Write the Unbalanced Equation: Begin by writing the chemical formulas for the acid and base reactants, and the salt and water products. For example, let's consider the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH):
HCl + NaOH → NaCl + H₂O
2. Identify the Ions: Break down the reactants into their constituent ions. In this case:
H⁺ + Cl⁻ + Na⁺ + OH⁻ → NaCl + H₂O
3. Combine Ions to Form Water and Salt: Notice that the H⁺ and OH⁻ ions combine to form water (H₂O). The remaining ions (Na⁺ and Cl⁻) form the salt, sodium chloride (NaCl).
4. Check for Balance: Count the number of atoms of each element on both sides of the equation. In this example, the equation is already balanced: one sodium (Na), one chlorine (Cl), two hydrogens (H), and one oxygen (O) on both sides.
5. Handle Polyprotic Acids and Bases: If the acid or base is polyprotic (contains multiple acidic or basic hydrogen/hydroxide ions), the balancing process becomes slightly more complex. For instance, the reaction between sulfuric acid (H₂SO₄) and potassium hydroxide (KOH):
H₂SO₄ + KOH → K₂SO₄ + H₂O
To balance this equation:
- Balance the sulfate ions (SO₄²⁻): You need two potassium ions (K⁺) to balance the sulfate anion's charge (2-). This requires two KOH units.
- Balance the hydrogen ions (H⁺): Two hydrogen ions from H₂SO₄ combine with two hydroxide ions (OH⁻) from two KOH units to form two water molecules (H₂O).
The balanced equation becomes:
H₂SO₄ + 2KOH → K₂SO₄ + 2H₂O
Examples of Balanced Neutralization Equations
Here are several examples illustrating the balancing process for different acid-base combinations:
Example 1: Strong Acid - Strong Base
Nitric acid (HNO₃) and potassium hydroxide (KOH):
HNO₃ + KOH → KNO₃ + H₂O (Already balanced)
Example 2: Strong Acid - Weak Base
Hydrochloric acid (HCl) and ammonia (NH₃):
HCl + NH₃ → NH₄Cl (Already balanced) Note: water isn't explicitly formed here because ammonia is a weak base that accepts a proton from the acid.
Example 3: Weak Acid - Strong Base
Acetic acid (CH₃COOH) and sodium hydroxide (NaOH):
CH₃COOH + NaOH → CH₃COONa + H₂O (Already balanced)
Example 4: Polyprotic Acid - Strong Base
Phosphoric acid (H₃PO₄) and calcium hydroxide Ca(OH)₂:
2H₃PO₄ + 3Ca(OH)₂ → Ca₃(PO₄)₂ + 6H₂O
Example 5: Diprotic Acid - Strong Base
Sulfuric acid (H₂SO₄) and sodium hydroxide (NaOH):
H₂SO₄ + 2NaOH → Na₂SO₄ + 2H₂O
Applications of Neutralization Reactions
Neutralization reactions have widespread applications in various fields:
- Titration: Neutralization reactions form the basis of acid-base titrations, a quantitative analytical technique used to determine the concentration of an unknown acid or base solution.
- Medicine: Antacids, used to neutralize excess stomach acid, rely on neutralization reactions.
- Environmental Remediation: Neutralization reactions are used to treat acidic or basic industrial wastewater before it's released into the environment.
- Chemical Synthesis: Neutralization reactions are crucial in various chemical syntheses to prepare salts and other compounds.
- Agriculture: Adjusting soil pH to optimal levels for plant growth often involves neutralization reactions.
Advanced Considerations
While the examples above cover common scenarios, some neutralization reactions involve more complex aspects:
- Amphoteric Substances: Some substances, like water and certain metal oxides/hydroxides, can act as both acids and bases. Balancing equations with amphoteric substances requires careful consideration of the reaction conditions.
- Buffer Solutions: Mixing weak acids or bases with their conjugate salts creates buffer solutions that resist changes in pH. Balancing equations involving buffer components requires a deeper understanding of equilibrium chemistry.
- Non-Aqueous Systems: Neutralization reactions can also occur in solvents other than water. Balancing equations in these systems requires awareness of the solvent's properties and its interactions with the acid and base.
Conclusion
Mastering the skill of balancing neutralization equations is essential for anyone studying or working in chemistry. This guide provides a comprehensive overview of the process, encompassing various types of acids and bases and highlighting different levels of complexity. Understanding these reactions is crucial for numerous practical applications across different scientific and industrial fields. By following the step-by-step approach outlined above and practicing with diverse examples, one can confidently balance neutralization equations and apply this knowledge to various chemical problems and scenarios. Remember to always double-check your balanced equation to ensure the number of atoms of each element is the same on both sides of the equation.
Latest Posts
Latest Posts
-
Why Are The Sedimentary Layers At Capitol Reef Tilted
Mar 17, 2025
-
How Do Spirochetes And Spirilla Differ
Mar 17, 2025
-
What Are Two Advantages Of Using Direct Mail And Catalogs
Mar 17, 2025
-
Postpurchase Cognitive Dissonance Is Especially Likely For Products That Are
Mar 17, 2025
-
Consider The Reaction Of 2 Methyl 1 3 Cyclohexadiene With Hcl
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Complete The Balanced Neutralization Equation For The Reaction Below . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
