Classify The Sugars As Either Aldoses Or Ketoses.
Holbox
Mar 27, 2025 · 6 min read
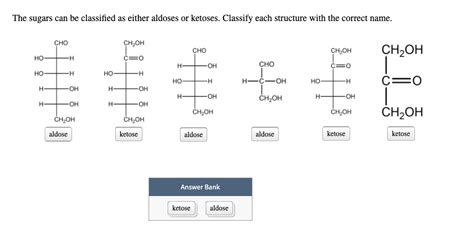
Table of Contents
- Classify The Sugars As Either Aldoses Or Ketoses.
- Table of Contents
- Classifying Sugars: Aldoses vs. Ketoses – A Comprehensive Guide
- Understanding the Basics: Monosaccharides
- Aldoses: The Aldehyde Sugars
- Common Aldoses:
- Chemical Properties of Aldoses:
- Ketoses: The Ketone Sugars
- Common Ketoses:
- Chemical Properties of Ketoses:
- Distinguishing Aldoses and Ketoses: Practical Methods
- Biological Significance of Aldoses and Ketoses:
- Conclusion: A Deeper Understanding of Sugar Chemistry
- Latest Posts
- Latest Posts
- Related Post
Classifying Sugars: Aldoses vs. Ketoses – A Comprehensive Guide
Sugars, the fundamental building blocks of carbohydrates, are ubiquitous in biological systems, playing crucial roles in energy storage, structural support, and cellular communication. Understanding their classification is essential for grasping their diverse functions and properties. This comprehensive guide delves into the classification of sugars as either aldoses or ketoses, exploring their structural differences, chemical properties, and biological significance.
Understanding the Basics: Monosaccharides
Before diving into the aldose-ketose classification, let's establish a foundational understanding of monosaccharides. Monosaccharides are the simplest form of carbohydrates, meaning they cannot be hydrolyzed into smaller sugar units. They are classified based on several key characteristics:
-
Number of carbon atoms: Monosaccharides are categorized based on the number of carbon atoms they possess. Common examples include trioses (3 carbons), tetroses (4 carbons), pentoses (5 carbons), hexoses (6 carbons), and heptoses (7 carbons).
-
Functional group: This is the critical distinction between aldoses and ketoses. The presence of either an aldehyde or a ketone functional group dictates their classification.
Aldoses: The Aldehyde Sugars
Aldoses are monosaccharides containing an aldehyde group (-CHO) at one end of their carbon chain. The aldehyde group is characterized by a carbon atom double-bonded to an oxygen atom and single-bonded to a hydrogen atom. This functional group is highly reactive and participates in numerous important biochemical reactions.
Common Aldoses:
-
Glyceraldehyde (Triose): The simplest aldose, possessing three carbon atoms. It serves as a crucial intermediate in various metabolic pathways. Its chirality (presence of a chiral carbon) is foundational to understanding the stereochemistry of sugars.
-
Erythrose (Tetrose): A four-carbon aldose with significant roles in the synthesis of certain amino acids.
-
Ribose (Pentose): A five-carbon aldose that forms the backbone of RNA (ribonucleic acid), a vital molecule in protein synthesis. Its derivative, deoxyribose, forms the backbone of DNA (deoxyribonucleic acid).
-
Glucose (Hexose): Perhaps the most important monosaccharide, glucose is the primary source of energy for most living organisms. It undergoes glycolysis, a crucial metabolic pathway that releases energy.
-
Galactose (Hexose): A structural isomer of glucose, galactose is found in lactose (milk sugar) and plays a significant role in brain development.
-
Mannose (Hexose): Another hexose isomer of glucose, mannose is a component of glycoproteins and polysaccharides, involved in cell signaling and immune responses.
Chemical Properties of Aldoses:
The aldehyde group in aldoses exhibits several characteristic chemical properties.
-
Oxidation: Aldoses can be easily oxidized, meaning they readily donate electrons. This property is exploited in various biochemical assays to detect and quantify aldoses. Benedict's solution and Fehling's solution are classic examples of reagents that detect reducing sugars, including aldoses.
-
Reduction: Aldoses can also be reduced, accepting electrons to form alditols. This process is crucial in certain metabolic pathways.
-
Formation of cyclic structures: In aqueous solutions, aldoses predominantly exist in cyclic forms (pyranose or furanose rings), owing to the reaction between the aldehyde group and a hydroxyl group on the carbon chain. This ring formation stabilizes the molecule.
Ketoses: The Ketone Sugars
Ketoses are monosaccharides containing a ketone group (=C=O) within their carbon chain, typically on the second carbon atom. The ketone group, featuring a carbon atom double-bonded to an oxygen atom, is also reactive but differs from the aldehyde group in its reactivity patterns.
Common Ketoses:
-
Dihydroxyacetone (Triose): The simplest ketose, a three-carbon molecule. It's an intermediate in glycolysis and other metabolic pathways.
-
Fructose (Hexose): A common ketose found in fruits and honey. Fructose is rapidly metabolized in the liver and is often sweeter than glucose. It forms a furanose ring structure in solution.
-
Sorbose (Hexose): A hexose ketose used in the production of vitamin C (ascorbic acid).
-
Xylulose (Pentose): A five-carbon ketose involved in the pentose phosphate pathway, a vital metabolic route for nucleotide biosynthesis.
Chemical Properties of Ketoses:
Ketoses, while sharing some similarities with aldoses, exhibit distinct chemical properties due to the presence of the ketone group.
-
Oxidation: Ketoses can also be oxidized, although typically less readily than aldoses. They can still undergo oxidation under specific conditions, such as in the presence of strong oxidizing agents.
-
Reduction: Similar to aldoses, ketoses can be reduced to form polyhydroxy alcohols (ketols).
-
Formation of cyclic structures: Like aldoses, ketoses predominantly exist in cyclic forms in solution, but their ring structures are slightly different. Fructose, for example, predominantly forms a five-membered furanose ring.
-
Isomerization: A unique characteristic of ketoses is their ability to isomerize into aldoses under certain conditions. This isomerization, often catalyzed by enzymes, is crucial in metabolic pathways where interconversion between aldoses and ketoses is necessary. For instance, fructose can isomerize to glucose and mannose.
Distinguishing Aldoses and Ketoses: Practical Methods
Several methods can be employed to differentiate between aldoses and ketoses:
-
Tollen's test: This test uses Tollen's reagent, which is a mild oxidizing agent. Aldoses, with their readily oxidizable aldehyde group, readily reduce Tollen's reagent, producing a silver mirror. Ketoses typically give a negative or weaker positive result.
-
Benedict's test/Fehling's test: These tests use copper(II) ions as an oxidizing agent. Aldoses reduce copper(II) ions to copper(I) ions, resulting in a color change (typically from blue to brick-red). Ketoses may give a positive result, but it's often slower and less intense than with aldoses. However, specific conditions can lead to a positive test in certain ketoses.
-
Chromatographic techniques: Methods like thin-layer chromatography (TLC) and high-performance liquid chromatography (HPLC) can separate and identify aldoses and ketoses based on their different polarities and retention times. These techniques offer high accuracy and resolution.
Biological Significance of Aldoses and Ketoses:
The distinction between aldoses and ketoses is not merely an academic exercise; it holds immense biological significance. The specific chemical properties and reactivity of these sugar types dictate their roles in various biological processes:
-
Energy Metabolism: Glucose (aldose) is the central molecule in energy metabolism, serving as the primary fuel for cellular respiration. Fructose (ketose) is also a crucial energy source, albeit metabolized differently.
-
Structural Components: Aldoses like ribose and deoxyribose are integral components of nucleic acids (RNA and DNA), the blueprints of life. Other sugars contribute to the structure of polysaccharides like cellulose (plant cell walls) and chitin (insect exoskeletons).
-
Cell Signaling: Many sugars are involved in cell-cell recognition and communication. Glycoproteins and glycolipids, which contain various aldoses and ketoses, play vital roles in immune responses, cell adhesion, and other cellular processes.
-
Metabolic Intermediates: Aldoses and ketoses serve as essential intermediates in numerous metabolic pathways, facilitating the synthesis and breakdown of carbohydrates, lipids, and other biomolecules.
Conclusion: A Deeper Understanding of Sugar Chemistry
The classification of sugars as either aldoses or ketoses is fundamental to understanding their chemical properties and biological functions. The presence of an aldehyde or ketone group dramatically influences their reactivity, leading to their diverse roles in energy metabolism, structural support, and cellular communication. Further research continues to unravel the intricate complexities of sugar chemistry, revealing their multifaceted roles in the intricate machinery of life. Knowing the distinctions between aldoses and ketoses is not merely a matter of academic interest but a key to understanding the fundamental processes underpinning life itself. The specific properties of these sugars – their reactivity, their ability to form cyclic structures, and their participation in metabolic pathways – are crucial for comprehending their wide-ranging biological significance. This comprehensive exploration of aldoses and ketoses provides a solid foundation for further study into the fascinating world of carbohydrate chemistry and its biological implications.
Latest Posts
Latest Posts
-
Multiple Sclerosis And Atherosclerosis Both Refer To
Mar 31, 2025
-
A Game Is Said To Be Fair If
Mar 31, 2025
-
Which Of The Following Is The Strongest Acid
Mar 31, 2025
-
Please Label The Circular Flow Diagram
Mar 31, 2025
-
Identify True Statements About The Si Unit Of Force
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Classify The Sugars As Either Aldoses Or Ketoses. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
