Arrange The Given Compounds Based On Their Relative Brønsted Acidities
Holbox
Mar 22, 2025 · 6 min read
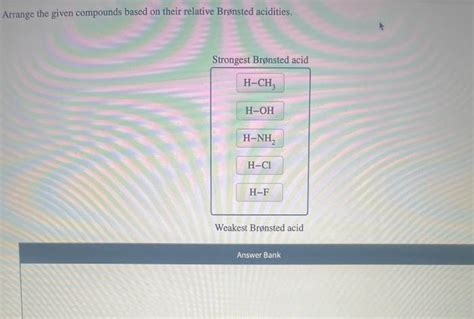
Table of Contents
- Arrange The Given Compounds Based On Their Relative Brønsted Acidities
- Table of Contents
- Arranging Compounds Based on Brønsted Acidity: A Comprehensive Guide
- Understanding Brønsted Acidity
- Factors Affecting Brønsted Acidity
- 1. Inductive Effects
- 2. Resonance Effects
- 3. Hybridization
- 4. Electronegativity
- 5. Solvent Effects
- Practical Application: Arranging Compounds Based on Acidity
- Advanced Considerations
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Arranging Compounds Based on Brønsted Acidity: A Comprehensive Guide
Understanding Brønsted acidity is crucial in chemistry. This guide delves deep into the concept, providing a structured approach to arranging compounds based on their relative Brønsted acidities. We'll explore the factors influencing acidity, including inductive effects, resonance, hybridization, and electronegativity, and provide practical examples to solidify your understanding. This detailed explanation will empower you to confidently predict and compare the acidities of various chemical compounds.
Understanding Brønsted Acidity
A Brønsted-Lowry acid is defined as a substance that donates a proton (H⁺). The strength of an acid depends on its willingness to donate this proton. A stronger acid readily donates its proton, while a weaker acid holds onto its proton more tightly. The relative acidity of compounds can be compared by considering the stability of the resulting conjugate base after proton donation. The more stable the conjugate base, the stronger the acid.
Factors Affecting Brønsted Acidity
Several key factors influence the stability of the conjugate base and consequently, the acidity of the compound. Let's examine them in detail:
1. Inductive Effects
Inductive effects describe the polarization of a sigma bond caused by the electronegativity difference between atoms. Electron-withdrawing groups (EWGs) like halogens (F, Cl, Br, I), nitro groups (NO₂), and cyano groups (CN) stabilize the negative charge on the conjugate base by pulling electron density away from the negatively charged atom. This stabilization makes the acid stronger. Conversely, electron-donating groups (EDGs) destabilize the negative charge, making the acid weaker.
Example: Compare the acidity of acetic acid (CH₃COOH) and trifluoroacetic acid (CF₃COOH). The three fluorine atoms in trifluoroacetic acid are highly electronegative EWGs. They pull electron density away from the carboxylate anion (conjugate base), stabilizing it significantly more than in the acetate anion. Therefore, trifluoroacetic acid is a much stronger acid than acetic acid.
2. Resonance Effects
Resonance involves the delocalization of electrons across multiple atoms. If the conjugate base can participate in resonance, the negative charge is spread over multiple atoms, significantly increasing its stability. This leads to a stronger acid.
Example: Compare the acidity of phenol (C₆H₅OH) and cyclohexanol (C₆H₁₁OH). The phenoxide ion (conjugate base of phenol) can participate in resonance, delocalizing the negative charge across the benzene ring. The cyclohexoxide ion (conjugate base of cyclohexanol) lacks this resonance stabilization. Consequently, phenol is a significantly stronger acid than cyclohexanol.
3. Hybridization
The hybridization of the atom bearing the acidic proton also plays a role. Atoms with higher s-character hold electrons more tightly. Therefore, acids with the acidic proton attached to an sp hybridized carbon are stronger than those with the proton attached to an sp² or sp³ hybridized carbon. This is because sp hybridized orbitals have more s-character (50%) compared to sp² (33%) and sp³ (25%).
Example: Acetylene (HC≡CH) is a stronger acid than ethylene (H₂C=CH₂) which is stronger than ethane (H₃C-CH₃). The acidic proton in acetylene is attached to an sp hybridized carbon, while in ethylene it's attached to an sp² hybridized carbon and in ethane to an sp³ hybridized carbon. The increased s-character in the sp hybridized carbon leads to greater electron density near the nucleus, making the proton more readily released.
4. Electronegativity
The electronegativity of the atom directly bonded to the acidic proton influences acidity. A more electronegative atom attracts electrons more strongly, stabilizing the negative charge on the conjugate base and enhancing the acid's strength.
Example: Consider the hydrohalic acids (HF, HCl, HBr, HI). Fluorine is the most electronegative element. While you might expect HF to be the strongest acid, the bond strength is also a factor. The H-F bond is very strong, making it difficult to release the proton. As we move down the group, the bond strength decreases (and the size of the halogen increases), making the proton release easier and the acid stronger. The trend observed is HI > HBr > HCl > HF. The stronger the electronegativity only stabilizes the anion after the proton has been released.
5. Solvent Effects
The solvent also affects the apparent acidity of a compound. Protic solvents, those capable of hydrogen bonding, can stabilize the conjugate base, increasing the acidity of the compound. Aprotic solvents, lacking the ability to form hydrogen bonds, have less impact on the acidity. These effects can often be subtle but are critical to consider in precise acidity comparisons.
Practical Application: Arranging Compounds Based on Acidity
Let's apply these principles to arrange a set of compounds based on their relative Brønsted acidities. To illustrate, let's consider the following compounds:
- Ethanol (CH₃CH₂OH)
- Water (H₂O)
- Acetic acid (CH₃COOH)
- Trichloroacetic acid (Cl₃CCOOH)
- Phenol (C₆H₅OH)
- Hydrochloric acid (HCl)
- Methane (CH₄)
Arranging these compounds in order of increasing acidity:
-
Methane (CH₄): Methane is the weakest acid among the given compounds. The conjugate base, CH₃⁻, is highly unstable due to the presence of a negatively charged carbon atom that is relatively electronegative, and its inability to form resonance.
-
Ethanol (CH₃CH₂OH): Ethanol is a weak acid. The ethoxide ion is more stable than the methanide ion due to better charge dispersal than the methanide ion, but still significantly less stable than the other conjugate bases in the list.
-
Water (H₂O): Water is a slightly stronger acid than ethanol. The hydroxide ion (OH⁻) is relatively stable due to the high electronegativity of oxygen, but less stable than carboxylates.
-
Phenol (C₆H₅OH): Phenol is a stronger acid than water due to resonance stabilization of the phenoxide ion. The negative charge is delocalized over the benzene ring.
-
Acetic acid (CH₃COOH): Acetic acid is a stronger acid than phenol. The carboxylate ion is further stabilized by resonance, and the electronegativity of the oxygen atoms helps to disperse the negative charge.
-
Trichloroacetic acid (Cl₃CCOOH): Trichloroacetic acid is a much stronger acid than acetic acid. The three chlorine atoms are electron-withdrawing groups, significantly stabilizing the conjugate base through an inductive effect.
-
Hydrochloric acid (HCl): Hydrochloric acid is the strongest acid in this list. The chloride ion (Cl⁻) is a very stable conjugate base due to the high electronegativity of chlorine and its large size, which helps to disperse the negative charge effectively.
Therefore, the complete order of increasing acidity is: CH₄ < CH₃CH₂OH < H₂O < C₆H₅OH < CH₃COOH < Cl₃CCOOH < HCl
Advanced Considerations
This analysis provides a fundamental understanding. More complex molecules may require a detailed consideration of steric effects, hydrogen bonding, and other subtle interactions to fully predict their relative acidities. Sophisticated computational methods are often employed for more accurate predictions in such cases.
Conclusion
Predicting the relative Brønsted acidity of compounds requires a comprehensive understanding of various factors, including inductive effects, resonance, hybridization, electronegativity, and solvent effects. By carefully analyzing these factors, we can confidently arrange compounds in order of increasing or decreasing acidity. This knowledge is fundamental to many areas of chemistry, including organic chemistry, biochemistry, and physical chemistry. This detailed guide provides a solid foundation for mastering this essential concept. Remember to always consider the interplay of these factors when making comparisons, as the relative importance of each can vary depending on the specific molecules involved.
Latest Posts
Latest Posts
-
Correctly Label The Features Of The Larynx
Mar 24, 2025
-
A Large Sunflower Population Is Established In A Field
Mar 24, 2025
-
Which Statement Concerning Rare Threatened Or Endangered Species Is True
Mar 24, 2025
-
Match Each Spinal Nerve With The Main Structures It Supplies
Mar 24, 2025
-
Which Of The Following Statements About Sexual Selection Is Correct
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Arrange The Given Compounds Based On Their Relative Brønsted Acidities . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
