Add Curved Arrows To Draw Step 1 Of The Mechanism
Holbox
Mar 19, 2025 · 6 min read
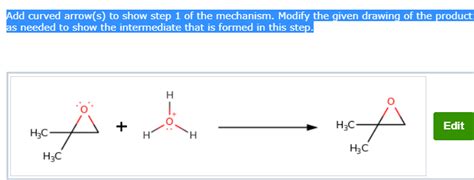
Table of Contents
Adding Curved Arrows to Draw Step 1 of a Reaction Mechanism: A Comprehensive Guide
Drawing reaction mechanisms using curved arrows is a fundamental skill in organic chemistry. These arrows represent the movement of electrons during a reaction, providing a visual roadmap of the bond-breaking and bond-forming processes. This guide will walk you through the intricacies of drawing curved arrows, focusing specifically on the crucial first step of a mechanism. We'll cover the rules, common mistakes, and provide numerous examples to solidify your understanding.
Understanding the Basics of Curved Arrows
Before diving into specific examples, let's establish the foundation. Curved arrows represent the flow of electrons, not the movement of atoms. A single-headed arrow (→) indicates the movement of one electron (a radical reaction), while a double-headed arrow (⇀) indicates the movement of two electrons (most common in ionic reactions). The tail of the arrow originates from the electron source (usually a lone pair, a pi bond, or a bond undergoing heterolytic cleavage), and the head points to the electron sink (usually an atom with an empty orbital or a bond undergoing formation).
Key Rules for Drawing Curved Arrows:
- Electron Source: The arrow always starts at a source of electrons – a lone pair, a pi bond, or a bond that is breaking heterolytically.
- Electron Sink: The arrowhead points towards the atom or bond that is receiving the electrons. This is often an electrophile (electron-deficient species) or a partially positive atom.
- One Electron at a Time: Avoid drawing arrows that transfer more than two electrons at once.
- Conservation of Charge: The overall charge of the system should remain consistent throughout the mechanism. Ensure that you correctly account for charges on all atoms.
- Clarity and Neatness: Draw clear, well-defined arrows that avoid ambiguity. Overlapping or poorly drawn arrows can make your mechanism difficult to understand.
Common Mistakes to Avoid When Drawing Curved Arrows
Many students struggle with drawing curved arrows, often making subtle but critical errors. Here are some common mistakes to watch out for:
- Incorrect Electron Source: Failing to identify the correct electron source can lead to an entirely incorrect mechanism. Pay close attention to lone pairs, pi bonds, and the possibility of bond heterolysis.
- Incorrect Electron Sink: Similarly, misidentifying the electron sink can derail the entire mechanism. Focus on the electrophilic sites (partially positive atoms or electron-deficient regions).
- Unbalanced Arrows: Don't forget to balance the movement of electrons. For every pair of electrons donated, there must be a pair of electrons accepted.
- Missing Formal Charges: Always include formal charges on the atoms involved. The omission of charges can easily lead to misinterpretations.
- Implied Arrows: Never assume what is happening. Always draw explicit arrows to show the movement of electrons, even if it seems intuitive.
Step-by-Step Examples of Drawing Step 1
Let's illustrate the process with several examples, focusing on drawing the first step of different reaction mechanisms.
Example 1: Nucleophilic Attack on a Carbonyl Group
This is a classic example of a nucleophilic addition reaction. Let's consider the reaction of a ketone with a Grignard reagent.
Step 1: The nucleophilic Grignard reagent (RMgX) attacks the electrophilic carbonyl carbon.
O O⁻
|| |
R₁-C-R₂ + R₃MgX -----> R₁-C-R₂
| |
R₃ MgX⁺
Curved Arrow Representation: A double-headed arrow originates from the lone pair on the carbon of the Grignard reagent and points to the carbonyl carbon. A second double-headed arrow shows the movement of the electrons in the carbonyl pi bond to the oxygen atom, creating a negatively charged oxygen.
Example 2: Proton Transfer
Acid-base reactions are ubiquitous in organic chemistry. Let's consider the deprotonation of an alcohol by a strong base.
Step 1: The base abstracts a proton from the alcohol.
R-O-H + ⁻OH -----> R-O⁻ + H₂O
Curved Arrow Representation: A double-headed arrow originates from the lone pair of the hydroxide ion (⁻OH) and points towards the hydrogen atom of the alcohol. A second double-headed arrow shows the movement of the electrons from the O-H bond to the oxygen atom of the alkoxide ion.
Example 3: SN1 Reaction: Formation of a Carbocation
The SN1 reaction (Substitution Nucleophilic Unimolecular) involves a carbocation intermediate. The first step is the rate-determining step, the dissociation of the leaving group to form a carbocation.
Step 1: The leaving group departs, forming a carbocation.
R₃C-X -----> R₃C⁺ + X⁻
Curved Arrow Representation: A single-headed arrow shows the movement of one electron of the C-X bond to the X, creating the negatively charged leaving group (X⁻). The other electron remains on the carbon, creating the carbocation.
Example 4: Electrophilic Aromatic Substitution: Attack of Electrophile
Electrophilic aromatic substitution reactions involve an electrophile attacking the aromatic ring. Let's consider nitration.
Step 1: The electrophile (NO₂⁺) attacks the aromatic ring.
Ar-H + NO₂⁺ -----> Ar-NO₂⁺ + H⁺
Curved Arrow Representation: A double-headed arrow from one of the pi bonds in the aromatic ring points to the electrophilic nitrogen of the nitronium ion (NO₂⁺). The pi electrons move to form a new bond with the electrophile. A positive charge now develops on the aromatic ring.
Example 5: Addition to an Alkene
Alkenes readily undergo addition reactions. Let's consider the addition of a proton (electrophile) to an alkene.
Step 1: Protonation of the alkene forms a carbocation.
R₂C=CR₂ + H⁺ -----> R₂C⁺-CHR₂
Curved Arrow Representation: A double-headed arrow originates from the pi bond of the alkene and points to the hydrogen. This forms a new sigma bond between the hydrogen and one of the carbons, creating a carbocation on the other carbon.
Practicing and Improving Your Skills
The key to mastering curved arrows is practice. Work through numerous examples in your textbook or online resources. Start with simpler reactions and gradually progress to more complex ones. Pay attention to detail, and don't hesitate to ask for help if you encounter difficulties. Compare your arrow pushing with solutions provided in textbooks or online resources to identify and correct any mistakes. Remember, consistent practice is the pathway to proficiency. Each example helps build a stronger understanding of electron flow and the principles behind reaction mechanisms.
Conclusion
Drawing curved arrows effectively is essential for understanding and representing reaction mechanisms in organic chemistry. By following the rules, carefully identifying the electron source and sink, and practicing regularly, you can improve your skills significantly. Remember to always maintain clarity and neatness in your drawings. The ability to accurately depict electron movement is critical for successful problem-solving and a deep understanding of organic chemistry. Through consistent practice and attention to detail, you’ll confidently navigate even the most complex reaction mechanisms. Mastering this fundamental skill will unlock a deeper comprehension of the fascinating world of chemical transformations.
Latest Posts
Latest Posts
-
What Is True Concerning Physical And Logical Topologies
Mar 19, 2025
-
A Coffee Producer Has Two Social Media Objectives
Mar 19, 2025
-
What Is The Medial Border Of The Highlighted Region Called
Mar 19, 2025
-
Select The Account Classification That Matches With The Description
Mar 19, 2025
-
An Inbound Sales Rep For A Digital
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Add Curved Arrows To Draw Step 1 Of The Mechanism . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
