A Ribbon Diagram Of A Zinc Metallo-beta-lactamase Protein Is Shown
Holbox
Mar 22, 2025 · 6 min read
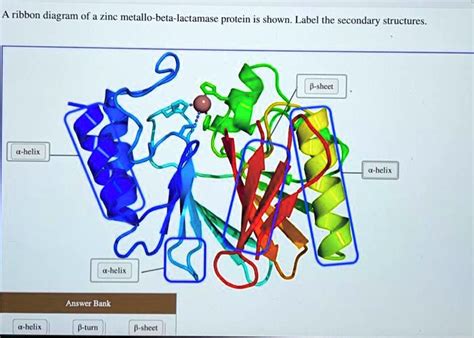
Table of Contents
- A Ribbon Diagram Of A Zinc Metallo-beta-lactamase Protein Is Shown
- Table of Contents
- A Ribbon Diagram of a Zinc Metallo-β-Lactamase Protein: A Deep Dive into Structure, Function, and Clinical Significance
- Understanding Zinc Metallo-β-Lactamase (MBL) Proteins
- The Role of Zinc Ions in MBL Function
- Deconstructing the Ribbon Diagram: Key Structural Features
- The Active Site Crevice: A Central Feature
- The α/β Hydrolase Fold: A Conserved Motif
- Loop Regions and Their Importance
- MBL Families and Subfamilies: Structural Diversity
- B1, B2, and B3 MBLs: A Structural Comparison
- Impact of Structural Variations on Antibiotic Resistance
- Clinical Significance and the Challenge of Antibiotic Resistance
- Carbapenem Resistance: A Major Concern
- The Urgent Need for Novel Therapeutics
- Future Directions and Research Opportunities
- Structure-Based Drug Design
- Developing MBL Inhibitors
- Exploring Alternative Therapeutic Strategies
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
A Ribbon Diagram of a Zinc Metallo-β-Lactamase Protein: A Deep Dive into Structure, Function, and Clinical Significance
A ribbon diagram offers a visually compelling representation of a protein's three-dimensional structure, highlighting its secondary structure elements like alpha-helices and beta-sheets. When applied to a zinc metallo-β-lactamase (MBL) protein, this visual tool unveils a complex architecture directly related to its potent antibiotic resistance mechanism. This article delves into the intricacies of MBLs, focusing on their structural features as revealed by ribbon diagrams, their functional mechanisms, and their significant clinical implications in the fight against bacterial infections.
Understanding Zinc Metallo-β-Lactamase (MBL) Proteins
β-lactamases are a broad class of enzymes produced by bacteria to inactivate β-lactam antibiotics, a crucial group of drugs used to treat bacterial infections. These antibiotics, including penicillin, cephalosporins, and carbapenems, target the bacterial cell wall synthesis process. MBLs represent a particularly challenging subclass because of their unique mechanism of action and broad substrate specificity. Unlike serine β-lactamases, which utilize a serine residue in their active site, MBLs employ one or two zinc ions (Zn²⁺) as essential cofactors within their active sites to catalyze the hydrolysis of the β-lactam ring. This hydrolytic process renders the antibiotic ineffective.
The Role of Zinc Ions in MBL Function
The zinc ions in the MBL active site are crucial for both substrate binding and the catalytic mechanism. One zinc ion acts as a Lewis acid, polarizing the carbonyl group of the β-lactam ring, making it more susceptible to nucleophilic attack. The second zinc ion stabilizes the transition state during the hydrolytic reaction. The precise coordination of these zinc ions by specific amino acid residues within the active site is essential for MBL activity. Variations in these coordinating residues can influence the enzyme's substrate specificity and its susceptibility to inhibitors. A ribbon diagram vividly illustrates the spatial arrangement of these key residues around the active site, providing critical insights into the enzyme's catalytic mechanism.
Deconstructing the Ribbon Diagram: Key Structural Features
A ribbon diagram of an MBL typically reveals a complex fold, often described as a mixed α/β structure. This means the protein is composed of both alpha-helices (represented by coils or spirals) and beta-sheets (represented by arrows or flat planes). The specific arrangement of these secondary structure elements varies slightly among different MBL families and subfamilies, but some common characteristics are observed:
The Active Site Crevice: A Central Feature
The active site is usually located in a deep cleft or crevice on the protein surface. This crevice provides a sheltered environment for substrate binding and catalysis, while also providing selectivity for β-lactam antibiotics over other molecules. The ribbon diagram clearly displays this crevice, showing how the alpha-helices and beta-sheets converge to form the walls of this crucial pocket. The precise positioning of specific amino acid side chains within this crevice, often highlighted by different colors in the ribbon diagram, emphasizes their role in substrate binding and catalysis.
The α/β Hydrolase Fold: A Conserved Motif
Many MBLs adopt a variation of the α/β hydrolase fold, a common structural motif found in various hydrolytic enzymes. This fold consists of a central β-sheet surrounded by α-helices. The ribbon diagram highlights this characteristic fold, illustrating how this conserved structural scaffold contributes to the stability and functionality of the enzyme. Variations within this fold across different MBLs can account for differences in their substrate specificity and inhibitor sensitivity.
Loop Regions and Their Importance
Loop regions, connecting the alpha-helices and beta-sheets, are often highly flexible and dynamic. These regions frequently participate in substrate binding and interaction with inhibitors. While less structured than the helices and sheets, loop regions are essential to MBL function. The ribbon diagram may highlight their location and flexibility, indicating their potential roles in modulating enzyme activity. Variations in loop sequence and length can be critical in determining MBL resistance to various inhibitors.
MBL Families and Subfamilies: Structural Diversity
While sharing a common α/β hydrolase-like fold, MBLs are categorized into various families and subfamilies based on their amino acid sequence similarity and subtle variations in their three-dimensional structures. These variations, often visible in their respective ribbon diagrams, can lead to differences in substrate specificity, inhibitor sensitivity, and overall catalytic efficiency.
B1, B2, and B3 MBLs: A Structural Comparison
The most prevalent MBL families are B1, B2, and B3. While each shares the core α/β hydrolase fold, subtle variations in the arrangement of helices and sheets, the length and conformation of loop regions, and the exact positioning of zinc-coordinating residues are readily apparent when comparing their ribbon diagrams. These seemingly minor structural differences can have significant functional consequences, influencing their ability to hydrolyze different β-lactam antibiotics and their susceptibility to different inhibitors.
Impact of Structural Variations on Antibiotic Resistance
Understanding these structural variations is crucial for developing novel inhibitors. Drugs designed to target the active site of one MBL subfamily may be ineffective against another due to structural differences in the active site pocket or surrounding regions. Ribbon diagrams, when compared across different MBL families, aid in visualizing these differences and guiding the rational design of new inhibitors.
Clinical Significance and the Challenge of Antibiotic Resistance
The emergence and spread of MBL-producing bacteria represent a significant threat to global health. These enzymes confer resistance to a wide range of β-lactam antibiotics, including carbapenems, which are often considered the last resort for treating serious infections caused by multi-drug resistant bacteria.
Carbapenem Resistance: A Major Concern
The ability of MBLs to hydrolyze carbapenems is particularly concerning because these antibiotics are typically reserved for infections resistant to other β-lactams. The spread of MBL-producing bacteria therefore necessitates the development of new therapeutic strategies to combat these infections.
The Urgent Need for Novel Therapeutics
Developing new antibiotics effective against MBL-producing bacteria is a crucial research priority. However, this task is complicated by the high degree of enzymatic plasticity and the broad substrate specificity of MBLs. Therefore, strategies are needed that either circumvent the action of the enzymes or target them directly. This includes the development of new β-lactam antibiotics that are less susceptible to hydrolysis, novel MBL inhibitors, and the exploration of alternative therapeutic approaches.
Future Directions and Research Opportunities
Ongoing research is focused on several promising areas:
Structure-Based Drug Design
Understanding the detailed three-dimensional structure of MBLs, as revealed by high-resolution techniques like X-ray crystallography and visualized in ribbon diagrams, enables structure-based drug design. This approach involves designing novel inhibitors that specifically target the active site of MBLs or other critical regions involved in substrate binding or catalysis.
Developing MBL Inhibitors
Researchers are actively developing various types of MBL inhibitors, including those that directly bind to the zinc ions in the active site, those that interfere with substrate binding, and those that target other crucial residues or regions on the enzyme.
Exploring Alternative Therapeutic Strategies
Beyond direct targeting of MBLs, other therapeutic strategies are being investigated. These include the exploration of molecules that can reactivate the activity of existing β-lactam antibiotics or the development of therapies that target other essential bacterial processes, thus bypassing the need to overcome MBL-mediated resistance.
Conclusion
The ribbon diagram, a powerful visualization tool, provides invaluable insights into the structure and function of zinc metallo-β-lactamases. Understanding the three-dimensional architecture of these enzymes is critical for developing strategies to combat the growing problem of antibiotic resistance. Further research utilizing advanced structural biology techniques, coupled with innovative drug design approaches, holds the key to overcoming the challenge posed by MBL-producing bacteria and preserving the efficacy of life-saving β-lactam antibiotics. The fight against antibiotic resistance requires a multi-faceted approach, and detailed structural analyses, as depicted in ribbon diagrams, are crucial elements in winning this battle.
Latest Posts
Latest Posts
-
Label The Photomicrograph Of Thick Skin
Mar 23, 2025
-
Human Pathogens Fall Into The Group
Mar 23, 2025
-
A Differentiation Strategy Works Best When A
Mar 23, 2025
-
Revenue Should Not Be Recognized Until
Mar 23, 2025
-
Engage Medical Surgical Alterations In Health
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about A Ribbon Diagram Of A Zinc Metallo-beta-lactamase Protein Is Shown . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
