Why Is The Freezing Point Of Xenon Higher Than Helium
Holbox
Mar 13, 2025 · 5 min read
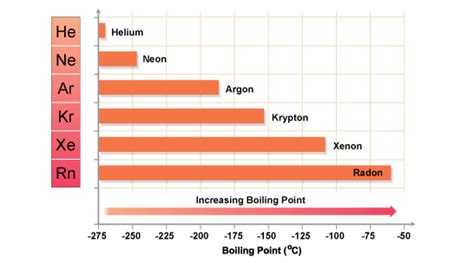
Table of Contents
Why is the Freezing Point of Xenon Higher Than Helium? A Deep Dive into Intermolecular Forces
The seemingly simple question of why xenon freezes at a much higher temperature than helium unveils a fascinating world of intermolecular forces and quantum mechanics. While both are noble gases, existing as monatomic gases at standard temperature and pressure, their vastly different freezing points stem from fundamental differences in their atomic structure and the resulting interactions between atoms. This article delves into the underlying physics, exploring the concepts of London Dispersion Forces (LDFs), atomic size, polarizability, and the quantum nature of helium to explain this intriguing phenomenon.
Understanding the Basics: Freezing Points and Noble Gases
Before delving into the specifics, let's establish a foundational understanding. The freezing point (or melting point) of a substance is the temperature at which it transitions from a liquid to a solid state, or vice versa, at standard atmospheric pressure. Noble gases, including helium and xenon, are characterized by their extremely low reactivity due to their complete valence electron shells. This stability means their atoms do not readily form covalent bonds with each other or other elements.
However, even noble gas atoms experience some degree of attraction, albeit weak, which is crucial in understanding their freezing points. These attractions are known as intermolecular forces.
The Dominant Force: London Dispersion Forces (LDFs)
The primary intermolecular force responsible for the weak attraction between noble gas atoms is the London Dispersion Force (LDF), also known as instantaneous dipole-induced dipole forces. These forces arise from temporary fluctuations in electron distribution around an atom.
Even though noble gas atoms have a symmetrical electron distribution on average, these distributions are not static. At any given instant, there's a probability of a slight asymmetry, creating a temporary, instantaneous dipole. This temporary dipole can then induce a dipole in a neighboring atom, leading to a weak attractive force between them.
The Role of Atomic Size and Polarizability
The strength of LDFs is directly related to the size and polarizability of the atom.
-
Atomic Size: Xenon atoms are significantly larger than helium atoms. Their larger electron clouds are further from the nucleus and are therefore more loosely held. This makes them more susceptible to temporary distortions and thus more polarizable.
-
Polarizability: Polarizability refers to the ease with which the electron cloud of an atom can be distorted to form a temporary dipole. Larger atoms like xenon are more polarizable because their outer electrons are further from the positively charged nucleus and are less tightly bound. This leads to stronger LDFs.
Consequently, the larger and more polarizable xenon atoms experience much stronger LDFs than the smaller and less polarizable helium atoms. These stronger intermolecular forces require more energy to overcome, resulting in a much higher freezing point for xenon.
Helium's Quantum Anomalies: Beyond Classical Physics
Helium's exceptionally low freezing point isn't solely explained by its small size and weak LDFs. Quantum mechanics plays a significant role.
Helium atoms are incredibly light, possessing very low mass. At very low temperatures, quantum effects become significant and significantly influence their behavior. Specifically, the zero-point energy of helium is relatively high compared to other atoms.
Zero-point energy is the minimum energy a quantum system can possess, even at absolute zero temperature. It's a direct consequence of the Heisenberg Uncertainty Principle, which states that we cannot simultaneously know both the position and momentum of a particle with perfect accuracy. This inherent uncertainty leads to a minimum vibrational energy, even at absolute zero.
In helium, this zero-point energy is sufficiently high to overcome the weak LDFs, preventing the atoms from readily solidifying even at extremely low temperatures. This quantum mechanical effect needs to be considered in understanding why helium remains a liquid even at temperatures approaching absolute zero. Only under immense pressure can the zero-point energy be overcome, allowing helium to solidify.
Comparing the Two: A Tabular Summary
To summarize the key differences that lead to the disparity in freezing points:
| Feature | Helium | Xenon |
|---|---|---|
| Atomic Size | Very Small | Large |
| Polarizability | Very Low | High |
| LDF Strength | Very Weak | Strong |
| Zero-Point Energy | Relatively High | Relatively Low |
| Freezing Point | -272.2 °C (-457.9 °F) | -111.8 °C (-169.2 °F) |
Further Considerations: Pressure and Isotopes
The freezing point of a substance can also be affected by pressure. While we have primarily discussed standard pressure, altering the pressure significantly impacts the freezing points of both helium and xenon. Increasing pressure generally favors the solid phase, as it increases the intermolecular interactions.
Isotopes, atoms of the same element with different numbers of neutrons, can also subtly affect freezing points. While the effect is typically small, variations in isotopic mass can influence the zero-point energy and slightly alter the freezing point.
Conclusion: A Tale of Size, Forces, and Quantum Mechanics
The dramatic difference in the freezing points of xenon and helium highlights the interplay of intermolecular forces, atomic size, polarizability, and quantum mechanics. While London Dispersion Forces are the dominant force in both cases, the significantly larger size and higher polarizability of xenon atoms lead to considerably stronger LDFs compared to helium. Moreover, the substantial zero-point energy of helium due to its light mass and quantum nature inhibits its solidification even at extremely low temperatures. Understanding these factors provides a comprehensive explanation for this seemingly simple yet fundamentally interesting question about the behavior of these noble gases. This comprehensive analysis, combining classical and quantum mechanical perspectives, offers a rich insight into the world of interatomic forces and the unique characteristics of matter at very low temperatures. The disparity in freezing points underscores the fundamental principles governing the physical states of matter and highlights the complexity of seemingly simple systems.
Latest Posts
Latest Posts
-
Which Of The Following Build New Strands Of Dna
Mar 13, 2025
-
Massage Mpntly Offers A Redcued Rate
Mar 13, 2025
-
A 2m Long Conducting Wire Is Formed Into A Sqire
Mar 13, 2025
-
Question New York Select All The Reagents
Mar 13, 2025
-
Being Ignored By Coworkers After Interrupting Them During A Meeting
Mar 13, 2025
Related Post
Thank you for visiting our website which covers about Why Is The Freezing Point Of Xenon Higher Than Helium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
