Question New York Select All The Reagents
Holbox
Mar 13, 2025 · 6 min read
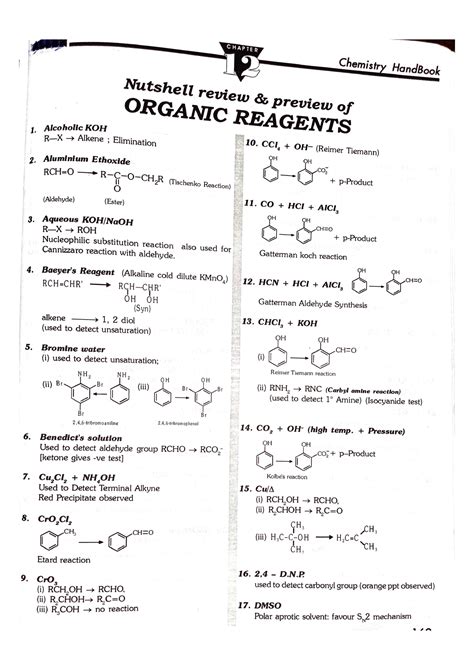
Table of Contents
- Question New York Select All The Reagents
- Table of Contents
- Question: New York Select All the Reagents
- Understanding the Challenge: Reagent Selection in Organic Synthesis
- Common Reagent Categories and Their Applications
- 1. Oxidizing Agents:
- 2. Reducing Agents:
- 3. Acids and Bases:
- 4. Nucleophiles and Electrophiles:
- 5. Protecting Groups:
- Strategic Approach to Solving "New York" Questions
- Example Problems and Solutions
- Conclusion: Mastering Reagent Selection
- Latest Posts
- Related Post
Question: New York Select All the Reagents
This article delves deep into the complexities of the "New York" question, a common challenge in organic chemistry focusing on reagent selection for specific transformations. We'll explore various reaction types, suitable reagents, and the underlying chemical principles governing their effectiveness. We'll also discuss strategic approaches to tackling such questions, emphasizing problem-solving techniques that will build a strong foundation in organic chemistry.
Understanding the Challenge: Reagent Selection in Organic Synthesis
The core of the "New York" question lies in your ability to select the appropriate reagents to achieve a desired transformation in organic synthesis. This requires a thorough understanding of:
- Functional Group Transformations: Recognizing and manipulating functional groups (e.g., alcohols, ketones, aldehydes, halides) is paramount. Each functional group has unique reactivity and requires specific reagents for transformation.
- Reaction Mechanisms: A deep grasp of reaction mechanisms (SN1, SN2, E1, E2, addition, elimination, etc.) allows you to predict the outcome of a reaction given a specific set of reagents and substrates.
- Reagent Properties: Understanding the reactivity and selectivity of various reagents (e.g., oxidizing agents, reducing agents, bases, acids, nucleophiles, electrophiles) is crucial for choosing the right one for a given transformation.
- Stereochemistry: Many organic reactions affect stereochemistry (chirality). Knowing how reagents affect stereochemistry is crucial for predicting the products of a reaction.
- Protecting Groups: Understanding the use of protecting groups is crucial when multiple functional groups are present and one needs to selectively modify only one of them. These protect reactive groups from unwanted transformations.
Common Reagent Categories and Their Applications
Let's explore some of the most frequently encountered reagent categories in organic synthesis:
1. Oxidizing Agents:
Oxidizing agents increase the oxidation state of a molecule. Common oxidizing agents and their applications include:
- Potassium Permanganate (KMnO₄): A strong oxidizing agent used for the oxidation of alkenes to diols, aldehydes to carboxylic acids, and primary alcohols to carboxylic acids. It's known for its vigorous oxidation power.
- Jones Reagent (CrO₃/H₂SO₄): Effectively oxidizes primary alcohols to carboxylic acids and secondary alcohols to ketones. It's a powerful, but potentially hazardous, oxidant.
- PCC (Pyridinium Chlorochromate): A milder oxidizing agent that converts primary alcohols to aldehydes and secondary alcohols to ketones, avoiding over-oxidation.
- Dess-Martin Periodinane (DMP): A very mild and selective oxidizing agent often used to convert primary alcohols to aldehydes and secondary alcohols to ketones, minimizing over-oxidation.
2. Reducing Agents:
Reducing agents decrease the oxidation state of a molecule. Examples include:
- Lithium Aluminum Hydride (LiAlH₄): A powerful reducing agent that reduces a wide range of functional groups, including esters, ketones, aldehydes, and carboxylic acids, to alcohols. It reacts violently with water.
- Sodium Borohydride (NaBH₄): A milder reducing agent that selectively reduces aldehydes and ketones to alcohols. It's less reactive than LiAlH₄ and can be used in protic solvents.
- Diisobutylaluminum Hydride (DIBAL-H): A selective reducing agent used to reduce esters to aldehydes and nitriles to aldehydes. It's often used at low temperatures to control reactivity.
3. Acids and Bases:
Acids and bases play crucial roles in many organic reactions, acting as catalysts or reagents:
- Strong Acids (e.g., H₂SO₄, HCl): Used in reactions like esterification, dehydration, and Friedel-Crafts alkylation.
- Strong Bases (e.g., NaOH, KOH, LDA): Used in reactions like deprotonation, elimination reactions, and nucleophilic substitution.
- Weak Acids (e.g., CH₃COOH): Often used as catalysts or to create specific reaction conditions.
- Weak Bases (e.g., pyridine): Used as bases or catalysts in many reactions, often to remove protons selectively.
4. Nucleophiles and Electrophiles:
Nucleophiles are electron-rich species that attack electron-deficient centers, while electrophiles are electron-deficient species that are attacked by nucleophiles. Examples include:
- Grignard Reagents (RMgX): Strong nucleophiles used to add carbon chains to carbonyl compounds.
- Organolithium Reagents (RLi): Similar to Grignard reagents, but even more reactive.
- Alkyl Halides: Can act as electrophiles in nucleophilic substitution reactions.
- Acyl Halides: React with nucleophiles to form esters, amides, and other derivatives.
5. Protecting Groups:
Protecting groups temporarily mask a functional group to prevent it from reacting during a desired transformation. Some commonly used protecting groups include:
- TBS (tert-Butyldimethylsilyl): Protects alcohols.
- MOM (Methoxymethyl): Protects alcohols.
- Benzyl (Bn): Protects alcohols and amines.
- Acetyl (Ac): Protects alcohols and amines.
Strategic Approach to Solving "New York" Questions
To effectively tackle "New York" questions, adopt a systematic approach:
-
Identify the Target Molecule: Carefully analyze the target molecule and identify the functional groups present. This helps you determine the necessary transformations.
-
Retrosynthetic Analysis: Work backward from the target molecule, identifying the key steps and the necessary reagents for each step. This breaks down the complex synthesis into manageable steps.
-
Consider Reaction Mechanisms: Understanding the reaction mechanism allows you to predict the outcome of a reaction and select the appropriate reagents.
-
Assess Reagent Compatibility: Ensure the chosen reagents are compatible with all functional groups present in the molecule. Some reagents may react with multiple functional groups, leading to unwanted side products.
-
Consider Stereochemistry: Be mindful of the stereochemical implications of the chosen reagents and reaction conditions.
Example Problems and Solutions
Let's examine a few examples to illustrate the practical application of reagent selection:
Example 1: Converting a primary alcohol to a carboxylic acid.
- Starting Material: Primary Alcohol
- Target Molecule: Carboxylic Acid
Solution: Strong oxidizing agents like Potassium Permanganate (KMnO₄) or Jones Reagent (CrO₃/H₂SO₄) are suitable for this transformation.
Example 2: Converting a ketone to a secondary alcohol.
- Starting Material: Ketone
- Target Molecule: Secondary Alcohol
Solution: Reducing agents like Sodium Borohydride (NaBH₄) or Lithium Aluminum Hydride (LiAlH₄) can achieve this reduction. NaBH₄ is preferred for its milder conditions and compatibility with other functional groups.
Example 3: Protecting an alcohol during a reaction.
- Starting Material: Molecule with an alcohol and another reactive functional group.
- Goal: React with the other functional group without affecting the alcohol.
Solution: Before performing the reaction on the other functional group, the alcohol needs to be protected using a protecting group like TBS (tert-butyldimethylsilyl) or MOM (methoxymethyl). After the desired reaction, the protecting group can be removed.
Conclusion: Mastering Reagent Selection
Mastering the art of reagent selection is fundamental to success in organic chemistry. This involves a combination of knowledge, problem-solving skills, and a systematic approach. By understanding the properties of various reagents and their mechanisms of action, and by employing retrosynthetic analysis, you can effectively tackle complex synthetic challenges and design efficient and selective chemical transformations. Consistent practice and a focus on understanding the underlying chemical principles are key to achieving proficiency in this critical area of organic chemistry. Remember to always prioritize safety and handle reagents with care.
Latest Posts
Related Post
Thank you for visiting our website which covers about Question New York Select All The Reagents . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.