Which Reaction Sequence Best Accomplishes This Transformation
Holbox
Mar 22, 2025 · 5 min read
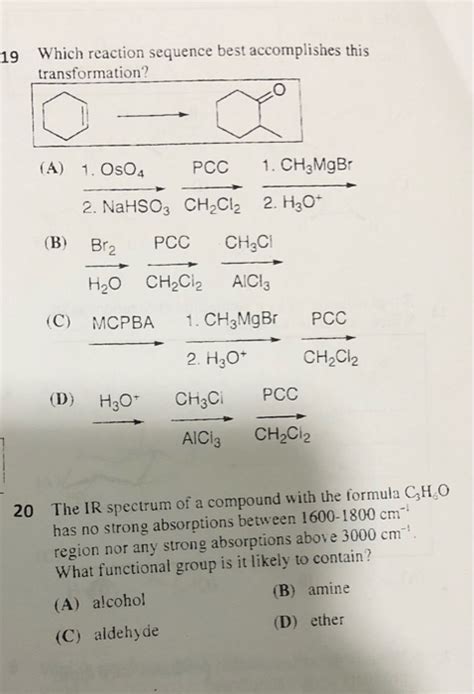
Table of Contents
Which Reaction Sequence Best Accomplishes This Transformation? A Comprehensive Guide
Choosing the optimal reaction sequence for a specific chemical transformation is a crucial skill in organic chemistry. It requires a deep understanding of reaction mechanisms, functional group reactivity, and the potential for side reactions. This article delves into the strategic considerations involved in selecting the best reaction sequence, focusing on the analysis of various possibilities and the evaluation of their efficiency and selectivity. We will explore several examples to illustrate the process and highlight the importance of careful planning.
Understanding the Problem: Defining the Starting Material and Target Molecule
Before we can even begin to consider reaction sequences, we must clearly define the starting material and the target molecule. This involves a thorough examination of their structures, identifying functional groups, and assessing the differences between them. This difference analysis dictates the transformations needed. Are we adding functional groups? Removing them? Altering their positions? All of these questions are crucial.
For example, let's consider a hypothetical transformation: converting a simple alkene to a 1,2-diol. This seemingly simple transformation actually presents several possible reaction pathways, each with its own advantages and drawbacks.
Exploring Potential Reaction Sequences: Analyzing Pathways and Reagents
For the alkene to 1,2-diol transformation, several routes are available:
1. Osmium Tetroxide (OsO₄) Oxidation: A Direct Approach
This is perhaps the most straightforward method. Osmium tetroxide is a powerful oxidant that directly adds two hydroxyl groups across the double bond in a syn addition. This means both hydroxyl groups end up on the same side of the molecule.
Mechanism: The reaction proceeds through a cyclic osmate ester intermediate, which is then hydrolyzed to yield the 1,2-diol.
Advantages: High yield, excellent stereoselectivity (syn addition), relatively clean reaction.
Disadvantages: Osmium tetroxide is highly toxic and expensive. The reaction often requires stoichiometric amounts of oxidant, making it less environmentally friendly. Furthermore, the reaction may be slower for sterically hindered alkenes.
2. Potassium Permanganate (KMnO₄) Oxidation: A Versatile Alternative
Potassium permanganate is another powerful oxidizing agent that can convert alkenes to 1,2-diols. However, the reaction conditions and the resulting stereochemistry can differ significantly depending on the reaction medium (acidic, basic, neutral).
Mechanism: The mechanism involves the formation of a cyclic manganate ester intermediate, similar to the osmium tetroxide reaction. However, the hydrolysis of this intermediate can be influenced by pH.
Advantages: KMnO₄ is cheaper and more readily available than OsO₄.
Disadvantages: Can lead to over-oxidation under certain conditions. The stereochemistry can be less predictable than with OsO₄. Can also produce manganese dioxide as a byproduct which can be difficult to remove.
3. Epoxidation followed by Ring Opening: A Multi-Step Strategy
This approach involves two steps: first, epoxidation of the alkene using a reagent such as mCPBA (meta-chloroperoxybenzoic acid) to form an epoxide; second, ring-opening of the epoxide using a nucleophile, such as water or a hydroxide ion, to yield the 1,2-diol.
Mechanism: Epoxidation proceeds via a concerted mechanism, resulting in the formation of a three-membered ring (epoxide). Ring-opening is typically an SN2 reaction, leading to inversion of configuration at one of the carbon atoms.
Advantages: mCPBA is less toxic than OsO₄. This method offers flexibility as the choice of nucleophile allows for some control over the stereochemistry of the final product (although it's not as precise as OsO4).
Disadvantages: A two-step process is generally less efficient than a one-step process. The reaction conditions for each step need to be carefully optimized. The ring opening step can be prone to side reactions, particularly in the presence of strong nucleophiles.
Comparative Analysis: Choosing the Best Reaction Sequence
Comparing the three approaches, the choice of the "best" reaction sequence depends on several factors:
- Cost and availability of reagents: KMnO₄ is the most cost-effective option.
- Toxicity of reagents: OsO₄ is highly toxic, posing significant safety concerns. mCPBA is less toxic but still requires careful handling.
- Stereoselectivity: OsO₄ provides the best stereoselectivity (syn addition).
- Reaction yield and efficiency: OsO₄ typically provides high yields, but the two-step epoxidation/ring opening method might have lower overall yield.
- Ease of purification: The presence of byproducts (like manganese dioxide from KMnO₄) can complicate purification.
For many applications, especially those emphasizing safety and cost-effectiveness, the epoxidation followed by ring-opening approach might be preferred despite its two-step nature. If perfect syn stereoselectivity is paramount, and cost is less of a concern, then OsO₄ oxidation becomes the clear choice, despite the toxicity. KMnO₄ oxidation offers a compromise between these two extremes, though the potential for over-oxidation and less predictable stereochemistry must be considered.
Expanding the Scope: More Complex Transformations
The principles outlined above apply equally well to more complex transformations. Consider the synthesis of a complex molecule with multiple functional groups. The strategy involves a step-by-step analysis:
-
Retrosynthetic Analysis: Work backward from the target molecule, identifying key disconnections and potential precursors. This helps to simplify the overall synthesis into manageable steps.
-
Functional Group Manipulation: Determine the necessary reactions to introduce or modify functional groups at each step. Consider the compatibility of reagents and reaction conditions. Avoiding side reactions is paramount.
-
Protecting Groups: When multiple functional groups are present, protecting groups are often necessary to selectively modify a specific functional group without affecting others. Careful selection of protecting groups and deprotection strategies is crucial.
-
Optimization and Iteration: The initial reaction sequence may need to be optimized through experimentation. This may involve adjusting reaction conditions, exploring alternative reagents, or even changing the overall synthetic strategy.
Conclusion: A Holistic Approach to Reaction Sequence Selection
Choosing the best reaction sequence is not a simple task; it is a multifaceted process requiring a thorough understanding of organic chemistry principles, reaction mechanisms, and practical considerations. The optimal sequence will balance factors such as yield, stereoselectivity, cost, safety, and ease of purification. By carefully analyzing the starting material, target molecule, and available reaction pathways, chemists can design efficient and effective synthetic routes to achieve their desired transformations. Retrosynthetic analysis, careful consideration of functional group compatibility, and judicious use of protecting groups are all essential tools in this crucial aspect of organic synthesis. The ability to strategically select the best reaction sequence is a testament to a chemist's expertise and experience. Careful planning and meticulous execution are key to success in the complex world of organic synthesis.
Latest Posts
Latest Posts
-
The General Model For Calculating A Quantity Variance Is
Mar 22, 2025
-
Large Circular Downwarped Structures Are Called
Mar 22, 2025
-
To Increase Value The Most Marketers Should
Mar 22, 2025
-
The Terms Run And Tumble Are Generally Associated With
Mar 22, 2025
-
The Allele For Black Noses In Wolves
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Which Reaction Sequence Best Accomplishes This Transformation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
