Which Of The Following Compounds Is Most Basic
Holbox
Mar 26, 2025 · 5 min read
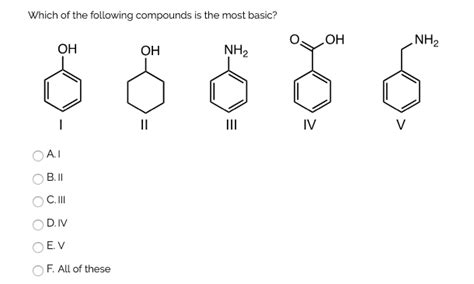
Table of Contents
- Which Of The Following Compounds Is Most Basic
- Table of Contents
- Which of the Following Compounds is Most Basic? A Deep Dive into Basicity
- Understanding Basicity: The Brønsted-Lowry and Lewis Theories
- Brønsted-Lowry Bases: Proton Acceptors
- Lewis Bases: Electron Pair Donors
- Factors Affecting Basicity: A Detailed Examination
- 1. Electronegativity: The Tug-of-War for Electrons
- 2. Inductive Effect: Neighborly Influence
- 3. Resonance: Electron Delocalization
- 4. Hybridization: Orbital Influence
- 5. Steric Hindrance: Bulky Neighbors
- Comparing Basicity: Practical Examples
- Advanced Considerations: Solvent Effects and Other Factors
- Conclusion: Predicting Basicity
- Latest Posts
- Latest Posts
- Related Post
Which of the Following Compounds is Most Basic? A Deep Dive into Basicity
Determining the relative basicity of different compounds is a crucial concept in chemistry, impacting numerous reactions and applications. This article will explore the factors influencing basicity and provide a comprehensive analysis of how to compare the basicity of various compounds. We'll delve into the nuances of different theories of basicity, focusing on practical examples to solidify understanding. Understanding this will enable you to confidently predict which compound will act as the strongest base in a given scenario.
Understanding Basicity: The Brønsted-Lowry and Lewis Theories
Before we dive into comparing specific compounds, let's establish a firm understanding of what constitutes a base. Two primary theories dominate the discussion of basicity: the Brønsted-Lowry theory and the Lewis theory.
Brønsted-Lowry Bases: Proton Acceptors
According to the Brønsted-Lowry theory, a base is a proton acceptor. This means it readily accepts a hydrogen ion (H⁺) from an acid. The stronger the base, the more readily it accepts a proton. This ability is directly related to the base's electron density and its ability to stabilize the resulting conjugate acid.
Lewis Bases: Electron Pair Donors
The Lewis theory provides a broader definition of a base. A Lewis base is defined as an electron pair donor. This definition encompasses Brønsted-Lowry bases, but also includes compounds that can donate electron pairs without necessarily accepting a proton. For instance, ammonia (NH₃) is both a Brønsted-Lowry and a Lewis base because it can donate its lone pair of electrons.
Factors Affecting Basicity: A Detailed Examination
Several key factors influence a compound's basicity:
1. Electronegativity: The Tug-of-War for Electrons
Electronegativity measures an atom's ability to attract electrons within a bond. Lower electronegativity generally leads to higher basicity. This is because atoms with lower electronegativity hold their electrons less tightly, making them more readily available to donate to a proton. Consider the hydroxide ion (OH⁻) compared to a fluoride ion (F⁻). Oxygen is less electronegative than fluorine, so the hydroxide ion is a stronger base.
2. Inductive Effect: Neighborly Influence
The inductive effect describes the influence of neighboring atoms or groups on the electron density of a molecule. Electron-donating groups increase electron density on the atom bearing the lone pair, enhancing basicity. Conversely, electron-withdrawing groups decrease electron density, reducing basicity. For example, consider comparing ethoxide (CH₃CH₂O⁻) and trifluoroethoxide (CF₃CH₂O⁻). The electron-withdrawing fluorine atoms in trifluoroethoxide reduce the electron density on the oxygen atom, making it a weaker base than ethoxide.
3. Resonance: Electron Delocalization
Resonance occurs when electrons can be delocalized over multiple atoms in a molecule. Resonance can significantly impact basicity. If the negative charge resulting from protonation can be delocalized through resonance, the resulting conjugate acid is stabilized, making the original compound a weaker base. Conversely, if resonance destabilizes the base but stabilizes the conjugate acid, the base will be stronger. Consider the acetate ion (CH₃COO⁻) – its negative charge is delocalized across two oxygen atoms, making it a weaker base than ethoxide.
4. Hybridization: Orbital Influence
The hybridization of the atom bearing the lone pair also influences basicity. Generally, higher s-character leads to lower basicity. This is because electrons in s-orbitals are held closer to the nucleus than those in p-orbitals. Therefore, sp hybridized atoms are less basic than sp³ hybridized atoms, which in turn are less basic than sp² hybridized atoms.
5. Steric Hindrance: Bulky Neighbors
Steric hindrance refers to the spatial arrangement of atoms or groups around the reaction center. Bulky groups surrounding the basic atom can hinder the approach of a proton, reducing basicity.
Comparing Basicity: Practical Examples
Let's analyze the basicity of some common compounds to illustrate the principles discussed above:
Example 1: Ammonia (NH₃) vs. Water (H₂O)
Ammonia is a stronger base than water. Nitrogen is less electronegative than oxygen, meaning it holds its lone pair of electrons less tightly and is more readily available for donation.
Example 2: Ethoxide (CH₃CH₂O⁻) vs. Methoxide (CH₃O⁻)
While both are strong bases, ethoxide is slightly weaker than methoxide. The ethyl group in ethoxide is slightly electron-donating, but it’s a relatively weak inductive effect compared to the size difference impacting steric hindrance around the oxygen.
Example 3: Aniline (C₆H₅NH₂) vs. Methylamine (CH₃NH₂)
Aniline is a much weaker base than methylamine. The phenyl group in aniline is electron-withdrawing due to resonance, reducing the electron density on the nitrogen atom and thus reducing its basicity.
Example 4: Acetate ion (CH₃COO⁻) vs. Hydroxide ion (OH⁻)
The acetate ion is a weaker base than the hydroxide ion. The negative charge on the acetate ion is delocalized across two oxygen atoms through resonance, stabilizing the ion and making it less likely to accept a proton.
Advanced Considerations: Solvent Effects and Other Factors
While the factors discussed above provide a good framework for understanding basicity, other considerations can significantly influence the relative basicity of compounds in specific scenarios:
-
Solvent Effects: The solvent in which the reaction takes place can dramatically alter the relative basicity of compounds. Protic solvents (those containing O-H or N-H bonds) can solvate bases, reducing their reactivity. Aprotic solvents generally lead to higher basicity.
-
Temperature: Temperature can also affect basicity. Generally, increased temperature can enhance the reactivity of bases.
Conclusion: Predicting Basicity
Predicting the relative basicity of compounds requires a careful consideration of several factors, including electronegativity, inductive effects, resonance, hybridization, steric hindrance, and solvent effects. By understanding these factors, one can accurately predict which of several compounds will be the strongest base under a specific set of conditions. This knowledge is crucial in organic chemistry, biochemistry, and many other areas of chemistry. This detailed analysis should equip you to confidently tackle problems comparing the basicity of various compounds. Remember that understanding the underlying principles is far more important than rote memorization of individual examples. Applying these principles will help you successfully navigate a wide array of chemical scenarios.
Latest Posts
Latest Posts
-
Which Of The Following Statements Is Most Correct
Mar 30, 2025
-
You Want The Title To Appear On Every Page
Mar 30, 2025
-
Correctly Label The Following External Anatomy Of The Anterior Heart
Mar 30, 2025
-
Find The Explicit General Solution To The Following Differential Equation
Mar 30, 2025
-
The Direct Carry Is Used To Transfer A Patient
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Compounds Is Most Basic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
