Which Formula Name Pair Is Incorrect
Holbox
Mar 13, 2025 · 5 min read
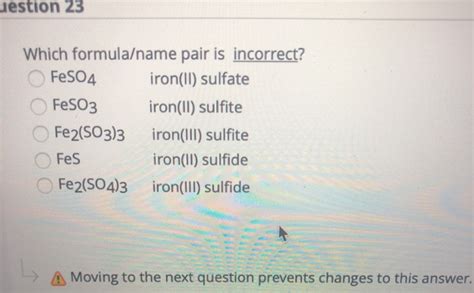
Table of Contents
Which Formula Name Pair is Incorrect? A Deep Dive into Chemical Nomenclature
The world of chemistry is built upon a foundation of precise language. Accurate naming of chemical compounds, a system known as chemical nomenclature, is crucial for unambiguous communication between scientists worldwide. A single misplaced letter or incorrect prefix can completely alter the meaning and properties of a described substance. This article delves into the nuances of chemical nomenclature, exploring common pitfalls and ultimately addressing the question: which formula-name pair is incorrect? We'll examine various types of inorganic and organic compounds, highlighting potential areas of confusion and providing a clear understanding of the rules governing chemical naming.
Understanding the Basics of Chemical Nomenclature
Before tackling specific examples, let's establish a firm understanding of the fundamental principles. Chemical nomenclature adheres to a set of rules developed by the International Union of Pure and Applied Chemistry (IUPAC). These rules ensure consistent and globally understood naming conventions. The core principles involve:
-
Identifying the constituent elements: The first step in naming any compound is to determine its constituent elements. This is often straightforward, but can become more complex with polyatomic ions or complex organic molecules.
-
Determining the oxidation state: Many elements can exist in multiple oxidation states, impacting their naming. Roman numerals are often used to clarify the oxidation state of the metal in a compound. For example, iron(II) oxide vs. iron(III) oxide.
-
Applying prefixes and suffixes: Prefixes (like mono-, di-, tri-) indicate the number of atoms of a particular element. Suffixes (-ide, -ite, -ate) indicate the type of anion or functional group present.
-
Prioritizing cation and anion placement: In ionic compounds, the cation (positively charged ion) is named first, followed by the anion (negatively charged ion).
Common Sources of Confusion in Chemical Nomenclature
Several aspects of chemical nomenclature can lead to errors. Let's examine some frequent areas of confusion:
-
Polyatomic ions: These are charged groups of atoms that act as single units. Remembering the names and charges of common polyatomic ions (e.g., sulfate, phosphate, nitrate) is essential. Mistakes often arise from incorrectly applying charges or forgetting their specific names.
-
Isomers: Isomers are molecules with the same molecular formula but different structural arrangements. This leads to different names, even though the elemental composition is identical. Careful consideration of the structural formula is required for correct naming.
-
Organic compounds: Organic nomenclature, particularly with complex molecules, can be exceptionally challenging. Understanding functional groups, parent chains, and substituents is paramount. The IUPAC system provides a systematic approach, but mastering it requires significant practice.
-
Hydrates: Compounds that incorporate water molecules into their crystal structure are called hydrates. Their names include prefixes indicating the number of water molecules (e.g., copper(II) sulfate pentahydrate). Inaccurate counting of water molecules leads to incorrect naming.
-
Acids: The nomenclature of acids depends on the anion they form. For example, HCl is hydrochloric acid, while H₂SO₄ is sulfuric acid. The rules for naming acids differ slightly from those for other compounds.
Examples of Incorrect Formula-Name Pairs
Let's examine a few hypothetical examples of incorrect formula-name pairs to illustrate common errors:
Example 1:
- Incorrect Pair: Fe₂O₃ – Iron(II) oxide
- Correct Pair: Fe₂O₃ – Iron(III) oxide
This error arises from incorrectly assigning the oxidation state of iron. In Fe₂O₃, iron has an oxidation state of +3, hence the correct name is iron(III) oxide.
Example 2:
- Incorrect Pair: NaCl₂ – Sodium chloride
- Correct Pair: NaCl – Sodium chloride
This demonstrates a common error in balancing charges. Sodium (Na) has a +1 charge, and chlorine (Cl) has a -1 charge. Therefore, the correct formula for sodium chloride is NaCl, not NaCl₂.
Example 3:
- Incorrect Pair: CaSO₄ – Calcium sulfide
- Correct Pair: CaSO₄ – Calcium sulfate
This error involves misidentifying the polyatomic ion. The SO₄²⁻ ion is sulfate, not sulfide (S²⁻).
Example 4: (Organic Chemistry Example)
- Incorrect Pair: CH₃CH₂CH₂OH – Propanal
- Correct Pair: CH₃CH₂CH₂OH – Propan-1-ol
This error confuses the functional group. Propan-1-ol has a hydroxyl (-OH) group, making it an alcohol, while propanal has an aldehyde group (-CHO)
Example 5: (Hydrate Example)
- Incorrect Pair: CuSO₄·5H₂O – Copper(II) sulfate dihydrate
- Correct Pair: CuSO₄·5H₂O – Copper(II) sulfate pentahydrate
This error incorrectly specifies the number of water molecules in the hydrate. The prefix "penta-" indicates five water molecules.
Advanced Considerations in Chemical Nomenclature
Beyond the basic principles, several advanced aspects of nomenclature require careful attention:
-
Coordination compounds: These compounds contain a central metal ion surrounded by ligands (molecules or ions bound to the metal). Their nomenclature involves specifying the ligands, their number, and the oxidation state of the metal.
-
Organic functional groups: A vast array of organic functional groups exists, each with its own naming conventions. Understanding these conventions is essential for accurately naming organic molecules.
-
Stereochemistry: The spatial arrangement of atoms in a molecule (stereochemistry) can significantly impact its properties and name. Cis-trans isomers and enantiomers are examples where stereochemistry is crucial in nomenclature.
Practical Tips for Mastering Chemical Nomenclature
-
Memorize common ions and their charges: A strong foundation in the charges of common ions is crucial for accurate formula writing and naming.
-
Practice, practice, practice: The best way to master chemical nomenclature is through consistent practice. Work through numerous examples and test your understanding.
-
Utilize online resources: Numerous online resources, including interactive exercises and quizzes, are available to aid in learning.
-
Consult a reliable textbook or reference: A comprehensive chemistry textbook can provide detailed explanations and examples.
-
Understand the IUPAC rules: Familiarity with the IUPAC rules is essential for accurate and consistent naming.
Conclusion: The Importance of Accuracy in Chemical Nomenclature
Accurate chemical nomenclature is not merely a matter of convention; it's fundamental to safety and clarity in the chemical sciences. Misnaming a compound can have severe consequences, particularly in industrial settings or laboratory research. By understanding the basic principles, practicing diligently, and referring to reliable resources, you can master the art of chemical nomenclature and ensure clear and unambiguous communication within the chemical community. The examples provided highlight the critical need for attention to detail and adherence to IUPAC guidelines. Continuous learning and practice are essential for avoiding errors and building a solid foundation in this vital area of chemistry. Remember, a seemingly small mistake in nomenclature can lead to significant errors in understanding and application.
Latest Posts
Latest Posts
-
When Should The Project Manager Prepare The Final Report
Mar 13, 2025
-
Whats The Average Height For A 6th Grade Boy
Mar 13, 2025
-
Using An Agent To Negotiate Shrinks The Zopa True False
Mar 13, 2025
-
What Is The Auto Mdix Feature
Mar 13, 2025
-
Write The Solutions That Can Be Read From The Matrix
Mar 13, 2025
Related Post
Thank you for visiting our website which covers about Which Formula Name Pair Is Incorrect . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
