Which Definition Best Describes The Term Activation Energy
Holbox
Mar 18, 2025 · 6 min read
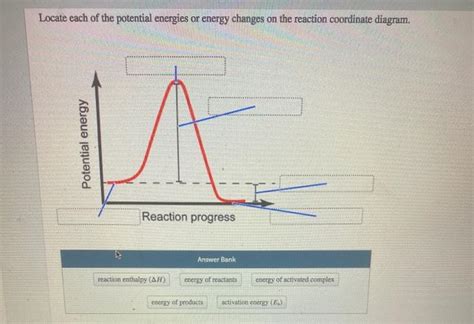
Table of Contents
Which Definition Best Describes the Term Activation Energy?
Activation energy: a term that frequently pops up in chemistry, biochemistry, and even material science. But what does it truly mean? Understanding activation energy is crucial for grasping many fundamental processes, from chemical reactions to enzyme function. This in-depth exploration will delve into various definitions, compare and contrast them, and ultimately pinpoint the most accurate and encompassing description of activation energy. We'll also explore its practical implications and significance across different scientific fields.
Activation Energy: A Conceptual Overview
Before diving into specific definitions, let's establish a foundational understanding. Activation energy is the minimum amount of energy required to initiate a chemical reaction. Think of it as the energy barrier that reactants must overcome to transform into products. Without sufficient activation energy, even highly favorable reactions (those with a negative Gibbs free energy change) will proceed at an infinitesimally slow rate or not at all.
Imagine a ball sitting at the top of a hill. To get it to roll down the other side (representing the completion of the reaction), you need to give it a push – that push is analogous to the activation energy. The height of the hill represents the energy barrier.
Common Definitions of Activation Energy and Their Shortcomings
Several definitions of activation energy exist, each with its nuances and limitations:
1. The minimum energy required for a reaction to occur. This is a simple and straightforward definition, easily understood by beginners. However, it lacks the crucial detail of the transition state, a high-energy intermediate state that reactants must achieve before forming products.
2. The energy difference between the reactants and the transition state. This definition is more precise as it highlights the role of the transition state. However, it's still incomplete because it doesn't account for the fact that activation energy is dependent on the reaction pathway (the specific mechanism by which the reaction occurs).
3. The energy needed to overcome the energy barrier separating reactants and products. This definition incorporates the concept of an energy barrier, accurately reflecting the energetic landscape of a reaction. It's better than the previous two but doesn't explicitly mention the transition state's importance.
4. The energy required to reach the activated complex. This is the most accurate definition. The activated complex (or transition state) is an unstable, high-energy intermediate species formed during the reaction. It represents the highest energy point along the reaction pathway. Reaching this activated complex requires overcoming the activation energy barrier.
The Superior Definition: A Deeper Dive
Based on the above analysis, the definition that most comprehensively and accurately describes activation energy is: The minimum amount of energy required for reactants to reach the activated complex (transition state), thus initiating the chemical reaction.
This definition encapsulates several crucial aspects:
-
Minimum Energy: It underscores that only a certain minimum energy is required; any energy exceeding this threshold will also allow the reaction to proceed.
-
Reactants: It specifically identifies the reactants as the species requiring the activation energy input.
-
Activated Complex (Transition State): It emphasizes the pivotal role of the transition state, the highest-energy point along the reaction pathway. The activated complex is not a stable intermediate but a fleeting species representing the point of no return between reactants and products.
-
Initiating the Chemical Reaction: It clearly states that overcoming the activation energy barrier is what triggers the reaction to progress towards the formation of products.
Factors Affecting Activation Energy
Several factors influence the activation energy of a reaction:
-
Nature of Reactants: The chemical properties of the reactants significantly impact the activation energy. Stronger bonds require more energy to break, leading to higher activation energies.
-
Reaction Mechanism: The specific pathway a reaction takes profoundly affects its activation energy. Different mechanisms will involve different transition states with varying energy levels.
-
Presence of a Catalyst: Catalysts dramatically reduce the activation energy by providing an alternative, lower-energy reaction pathway. They achieve this by either stabilizing the transition state or forming intermediate complexes with reactants.
-
Temperature: Higher temperatures increase the kinetic energy of reactant molecules, leading to a greater proportion of molecules possessing sufficient energy to surpass the activation energy barrier and react. This explains why increasing temperature typically accelerates reaction rates.
-
Surface Area (for heterogeneous reactions): In reactions occurring at a surface (e.g., catalysis on a solid surface), increasing the surface area provides more sites for reactant interaction, potentially lowering the activation energy.
Activation Energy in Different Scientific Fields
The concept of activation energy is not confined to just chemistry. Its implications extend widely across various scientific disciplines:
1. Biochemistry: Enzyme catalysis relies heavily on the reduction of activation energy. Enzymes bind to substrates, creating a microenvironment that stabilizes the transition state and significantly lowers the energy barrier for the reaction. This allows biochemical reactions to proceed at physiologically relevant rates.
2. Materials Science: Activation energy plays a key role in understanding material properties, particularly in processes like diffusion, sintering, and phase transformations. The activation energy for these processes reflects the energy barrier for atomic or molecular movement.
3. Environmental Science: Activation energy is crucial in understanding the kinetics of environmental processes, such as atmospheric reactions and pollutant degradation. The rate of these reactions is highly dependent on the activation energy and environmental factors like temperature and sunlight.
4. Physics: In many physical processes, such as crystal growth or nucleation, the concept of activation energy can be applied to describe the energy needed to overcome barriers in forming new phases or structures.
Practical Implications of Understanding Activation Energy
Understanding activation energy has far-reaching practical consequences:
-
Catalysis Development: Scientists design catalysts to selectively lower the activation energy of specific reactions, enabling efficient and sustainable industrial processes.
-
Drug Design: The activation energy of biological reactions is a critical factor in drug design. Drugs often act by either inhibiting or activating enzymes by modifying their activation energy.
-
Material Synthesis: Manipulating activation energy through process control is crucial in materials science to synthesize materials with desired properties.
-
Environmental Remediation: Understanding activation energy helps in designing efficient strategies for removing pollutants from the environment.
Conclusion: The Importance of Precision in Defining Activation Energy
While several definitions attempt to describe activation energy, using the most comprehensive and accurate definition – the minimum amount of energy required for reactants to reach the activated complex (transition state), thus initiating the chemical reaction – provides the clearest and most insightful understanding. This definition avoids oversimplification and encompasses the essential role of the transition state in the reaction mechanism.
The implications of understanding activation energy extend far beyond the realms of basic chemistry, playing a critical role in various scientific and technological advancements. The more accurately we define and understand this fundamental concept, the better equipped we are to harness its power for innovation and problem-solving across a wide array of scientific fields.
Latest Posts
Latest Posts
-
An Organization That Pursues A Single Product Strategy
Mar 18, 2025
-
A Pizza Restaurant Buys Pre Sliced Mushrooms
Mar 18, 2025
-
Art Labeling Activity Overview Of The Cardiac Conduction System
Mar 18, 2025
-
Copy And Paste Or Type Your Submission Right Here
Mar 18, 2025
-
Social Media Marketing Goals Must Be Flexible Because
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Which Definition Best Describes The Term Activation Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
