Which Chemical Activates The Transformation Of Trypsinogen To Trypsin
Holbox
Mar 21, 2025 · 5 min read
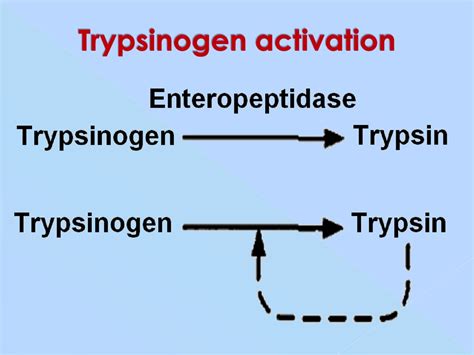
Table of Contents
- Which Chemical Activates The Transformation Of Trypsinogen To Trypsin
- Table of Contents
- Which Chemical Activates the Transformation of Trypsinogen to Trypsin?
- The Role of Enteropeptidase (Enterokinase)
- The Mechanism of Activation
- Significance of the Specific Cleavage Site
- Autocatalytic Activation of Trypsinogen
- The Significance of Autocatalysis
- Regulation of Autocatalysis
- Other Potential Activators
- Importance of Tight Regulation: Pancreatitis
- Mechanisms of Pancreatitis Development
- Role of Trypsin Inhibitors
- Clinical Implications and Therapeutic Strategies
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Which Chemical Activates the Transformation of Trypsinogen to Trypsin?
The conversion of trypsinogen to trypsin is a crucial step in the digestive process, a cascade reaction tightly regulated to prevent premature activation and self-digestion of the pancreas. Understanding this activation mechanism is fundamental to comprehending digestive health and various pancreatic pathologies. This article will delve into the chemical intricacies of this transformation, exploring the key players involved, the mechanisms of activation, and the implications of dysregulation.
The Role of Enteropeptidase (Enterokinase)
The primary chemical responsible for activating trypsinogen is enteropeptidase, also known as enterokinase. This serine protease is located in the brush border of the intestinal mucosa, specifically the duodenum. Enteropeptidase is a strategically placed enzyme; its location ensures that trypsinogen activation occurs only after the pancreatic secretions reach the small intestine, preventing premature activation within the pancreas itself.
The Mechanism of Activation
Enteropeptidase cleaves a specific peptide bond in trypsinogen, specifically at the lysine-isoleucine bond (Lys-Ile) located at the N-terminal region. This cleavage removes a hexapeptide from the trypsinogen molecule. This removal exposes the active site of the enzyme, transforming the inactive zymogen (trypsinogen) into the active protease, trypsin. The newly formed trypsin then exhibits autocatalytic activity, meaning it can catalyze the conversion of more trypsinogen molecules into trypsin. This autocatalytic process amplifies the initial activation event triggered by enteropeptidase, leading to a rapid and efficient cascade of trypsin generation.
Significance of the Specific Cleavage Site
The precise location of the cleavage site by enteropeptidase is crucial. The removal of the hexapeptide induces a conformational change in the trypsinogen molecule, allowing the essential catalytic residues to align correctly within the active site. This structural rearrangement is essential for trypsin's catalytic activity. The specificity of enteropeptidase for this particular bond ensures that only trypsinogen is activated, preventing unintended activation of other zymogens.
Autocatalytic Activation of Trypsinogen
While enteropeptidase initiates the process, trypsin itself can further activate trypsinogen. This autocatalytic activation creates a positive feedback loop, amplifying the conversion of trypsinogen to trypsin and ensuring a sufficient concentration of trypsin for efficient protein digestion in the small intestine.
The Significance of Autocatalysis
Autocatalysis is a significant element in the amplification of trypsin activation. A relatively small amount of trypsin initially generated by enteropeptidase can rapidly activate a large pool of trypsinogen, ensuring a quick and efficient digestive response. This mechanism is vital for effective protein digestion, considering the vast amount of protein that needs to be broken down in the small intestine.
Regulation of Autocatalysis
Despite its crucial role, autocatalysis needs to be precisely regulated to prevent uncontrolled trypsin activation. This regulation is achieved through several mechanisms, including the limited availability of trypsinogen and the presence of trypsin inhibitors. Dysregulation of this autocatalytic process can lead to severe complications, as we will explore further below.
Other Potential Activators
Although enteropeptidase is the primary activator, research suggests that other proteases might contribute to trypsinogen activation under specific circumstances. These include:
-
Mast cell chymases: These proteases are found in mast cells and have been implicated in trypsinogen activation, potentially contributing to pancreatitis. However, their role in physiological trypsinogen activation is less significant than enteropeptidase.
-
Bacterial proteases: Certain bacterial proteases present in the gut could theoretically activate trypsinogen. However, this mechanism is mostly associated with pathological conditions and is not a significant pathway in normal digestion.
The contribution of these alternative activators is generally considered less significant compared to the primary role of enteropeptidase and the autocatalytic activation by trypsin itself.
Importance of Tight Regulation: Pancreatitis
The tightly regulated activation of trypsinogen is crucial for preventing pancreatitis, a severe inflammatory condition of the pancreas. Premature activation of trypsinogen within the pancreas leads to the autodigestion of pancreatic tissues, triggering a cascade of inflammatory responses resulting in significant pain and potential organ damage.
Mechanisms of Pancreatitis Development
Several factors contribute to the development of pancreatitis, often involving a disruption in the balance of trypsinogen activation and inhibition. These factors include:
- Genetic mutations: Mutations in genes encoding trypsinogen or its inhibitors can lead to increased trypsin activity and increased risk of pancreatitis.
- Alcohol abuse: Alcohol consumption is a major risk factor for pancreatitis, possibly through its effects on intracellular calcium levels and causing premature trypsinogen activation.
- Gallstones: Gallstones can block the pancreatic duct, causing a build-up of pancreatic enzymes, including trypsinogen, increasing the likelihood of autoactivation.
- Infections: Certain infections can trigger inflammation and premature trypsinogen activation within the pancreas.
Role of Trypsin Inhibitors
The pancreas naturally produces trypsin inhibitors, such as pancreatic secretory trypsin inhibitor (PSTI), which plays a crucial role in preventing premature trypsin activation. PSTI binds to trypsin, inhibiting its activity and preventing autodigestion. Deficiencies or dysfunction of these inhibitors can contribute to the development of pancreatitis. The importance of PSTI in preventing autodigestion highlights the critical need for multiple levels of regulation to ensure that trypsin activation only occurs at the appropriate time and location.
Clinical Implications and Therapeutic Strategies
Understanding the precise mechanism of trypsinogen activation is crucial for developing therapeutic strategies for pancreatitis. Research focuses on:
- Trypsin inhibitor therapies: Developing more effective trypsin inhibitors to prevent or mitigate the effects of premature trypsin activation.
- Targeting enteropeptidase: Developing strategies to inhibit enteropeptidase activity in cases of excessive trypsinogen activation.
- Gene therapy: Investigating gene therapy approaches to correct genetic mutations that increase the risk of pancreatitis.
Current treatments for pancreatitis primarily focus on managing the symptoms and complications, such as pain relief, fluid management, and infection control. Further research into the precise mechanisms of trypsinogen activation and its regulation will undoubtedly lead to more targeted and effective therapies for this debilitating condition.
Conclusion
The transformation of trypsinogen to trypsin is a tightly regulated process vital for digestion and overall health. Enteropeptidase plays the crucial initiating role, triggering a cascade of events including autocatalytic amplification. The specific cleavage site and the subsequent conformational change are essential for trypsin's activity. The stringent regulation of this process, involving trypsin inhibitors and the strategic location of enteropeptidase, prevents premature activation and the devastating consequences of pancreatitis. Future research into the intricacies of this biochemical cascade will continue to refine our understanding of digestive health and provide avenues for developing more effective therapies for pancreatic diseases. The precise regulation and amplification of trypsin activation underscore the complexity and elegance of biological processes that maintain homeostasis and support essential life functions. Further exploration of this critical pathway promises to yield significant advancements in our understanding and treatment of pancreatic disorders.
Latest Posts
Latest Posts
-
There Are Almost 500 Naturally Occurring
Mar 28, 2025
-
Process Capability Compares Process Variability To The Tolerances
Mar 28, 2025
-
Do Black People Have Extra Calf Muscles
Mar 28, 2025
-
Assuming A Speculator Believes That The Canadian Dollar
Mar 28, 2025
-
The Figure Depicts A Situation Where
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Which Chemical Activates The Transformation Of Trypsinogen To Trypsin . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
