When Calcium Ions Enter The Synaptic Terminal
Holbox
Mar 24, 2025 · 6 min read
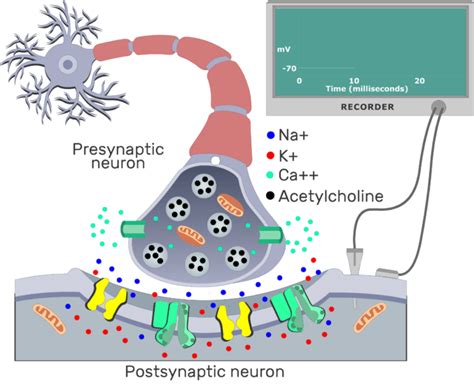
Table of Contents
- When Calcium Ions Enter The Synaptic Terminal
- Table of Contents
- When Calcium Ions Enter the Synaptic Terminal: A Deep Dive into Neurotransmission
- The Synaptic Terminal: A Molecular Machine
- The Arrival of the Action Potential: Setting the Stage for Calcium Influx
- The Role of Voltage-Gated Calcium Channels (VGCCs)
- Calcium's Role in Neurotransmitter Release: Exocytosis Unleashed
- The SNARE Complex: Orchestrating Vesicle Fusion
- The Kiss-and-Run Hypothesis: A More Subtle Release Mechanism
- Regulation of Calcium Influx: Fine-Tuning Neurotransmission
- Consequences of Dysfunctional Calcium Influx: Implications for Neurological Disorders
- Conclusion: A Complex Dance of Ions and Molecules
- Latest Posts
- Latest Posts
- Related Post
When Calcium Ions Enter the Synaptic Terminal: A Deep Dive into Neurotransmission
The precise orchestration of neuronal communication forms the bedrock of our thoughts, actions, and sensations. This communication, known as neurotransmission, hinges on a critical event: the influx of calcium ions (Ca²⁺) into the presynaptic terminal. This influx acts as the crucial trigger for the release of neurotransmitters, the chemical messengers that bridge the synaptic cleft and transmit signals to the postsynaptic neuron. Understanding the intricacies of calcium ion entry into the synaptic terminal is paramount to comprehending the fundamental mechanisms of the nervous system, and its malfunctions in neurological disorders.
The Synaptic Terminal: A Molecular Machine
Before delving into the calcium influx, let's establish a clear understanding of the synaptic terminal, the site where this pivotal event takes place. The presynaptic terminal, also known as the axon terminal or bouton, is the specialized ending of an axon, the long, slender projection of a neuron. It's a highly organized structure packed with intricate molecular machinery crucial for neurotransmitter release. Within the terminal, we find:
-
Synaptic vesicles: These small membrane-bound sacs store neurotransmitters, the chemical messengers that transmit signals across the synapse. They are strategically positioned near the presynaptic membrane, ready for release.
-
Voltage-gated calcium channels (VGCCs): These are the key players in our story. These transmembrane proteins act as selective gates, allowing the entry of calcium ions into the presynaptic terminal in response to changes in the membrane potential. Their precise location and type significantly influence neurotransmitter release.
-
Docking proteins: These molecules mediate the precise positioning of synaptic vesicles at the active zones, the specialized regions of the presynaptic membrane where neurotransmitter release occurs. They ensure efficient vesicle fusion and neurotransmitter exocytosis.
-
Proteins involved in vesicle fusion and recycling: A complex machinery of proteins ensures the fusion of synaptic vesicles with the presynaptic membrane, releasing their contents into the synaptic cleft, and the subsequent recycling of the vesicle membrane.
The Arrival of the Action Potential: Setting the Stage for Calcium Influx
The process begins with the arrival of an action potential, a rapid electrical signal, at the presynaptic terminal. This electrical signal is a wave of depolarization – a change in the membrane potential from negative to positive – that propagates along the axon. As the action potential reaches the terminal, it triggers the opening of voltage-gated calcium channels (VGCCs).
The Role of Voltage-Gated Calcium Channels (VGCCs)
The VGCCs are exquisitely sensitive to changes in membrane potential. When the membrane depolarizes, the VGCCs undergo a conformational change, opening their pores and allowing the influx of extracellular calcium ions. The concentration gradient of calcium ions, significantly higher outside the neuron than inside, drives this influx.
Several types of VGCCs exist, categorized into different families (e.g., P/Q-type, N-type, R-type, L-type) based on their biophysical properties, voltage sensitivity, and pharmacological sensitivity. The specific types of VGCCs present at a particular synapse influence the kinetics and amount of neurotransmitter released. For instance, P/Q-type VGCCs are particularly crucial for fast synaptic transmission at many synapses.
Calcium's Role in Neurotransmitter Release: Exocytosis Unleashed
The increase in intracellular calcium concentration, resulting from the opening of VGCCs, is the critical trigger for neurotransmitter release. Calcium ions bind to various proteins, initiating a cascade of events that lead to the fusion of synaptic vesicles with the presynaptic membrane.
The SNARE Complex: Orchestrating Vesicle Fusion
The process of vesicle fusion is elegantly orchestrated by a protein complex called SNARE (soluble NSF attachment protein receptor). This complex involves proteins on both the vesicle membrane (v-SNAREs) and the presynaptic membrane (t-SNAREs). Synaptotagmin, a calcium-binding protein, is crucial for this process. Upon calcium influx, synaptotagmin binds calcium ions, triggering a conformational change that promotes the interaction between v-SNAREs and t-SNAREs. This interaction brings the vesicle membrane close to the presynaptic membrane, leading to membrane fusion and the release of neurotransmitters into the synaptic cleft.
The Kiss-and-Run Hypothesis: A More Subtle Release Mechanism
While the full fusion of vesicles is the dominant model of neurotransmitter release, there's increasing evidence supporting the "kiss-and-run" hypothesis. In this model, the vesicle transiently interacts with the presynaptic membrane, releasing a fraction of its contents, before retracting back into the cytoplasm. This mechanism may be particularly relevant under certain conditions or at specific synapses.
Regulation of Calcium Influx: Fine-Tuning Neurotransmission
The precise regulation of calcium influx is crucial for maintaining the fidelity of neuronal signaling. Several mechanisms contribute to this regulation:
-
Calcium channel inactivation: After opening, VGCCs can inactivate, limiting the duration of calcium entry. This inactivation prevents excessive neurotransmitter release.
-
Calcium buffering: The cell utilizes various calcium-binding proteins (e.g., calbindin, parvalbumin) to quickly sequester calcium ions, preventing prolonged elevations in intracellular calcium concentration. This buffering helps to prevent excessive calcium-dependent signaling.
-
Calcium extrusion: Specialized pumps actively remove calcium ions from the presynaptic terminal, restoring baseline calcium levels and preparing the terminal for subsequent rounds of neurotransmitter release.
-
Presynaptic autoreceptors: Some neurons possess autoreceptors, specialized receptors that respond to the released neurotransmitters. Activation of these receptors can inhibit further neurotransmitter release by reducing calcium influx or affecting the SNARE complex.
Consequences of Dysfunctional Calcium Influx: Implications for Neurological Disorders
Dysfunction in calcium signaling at the presynaptic terminal can have severe consequences, leading to various neurological disorders. These dysfunctions can result from:
-
Mutations in VGCCs: Mutations affecting the structure or function of VGCCs can alter calcium influx, leading to impairments in neurotransmitter release. This has been implicated in several neurological conditions, including epilepsy and ataxia.
-
Disruptions in calcium buffering: Impairments in calcium buffering mechanisms can lead to prolonged elevations in intracellular calcium concentration, potentially triggering excitotoxicity—a process that can damage neurons through excessive stimulation. This is implicated in stroke and neurodegenerative diseases.
-
Altered SNARE complex function: Defects affecting the SNARE complex can compromise vesicle fusion and neurotransmitter release, leading to various neurological deficits. Botulinum toxin, for example, exerts its paralytic effect by cleaving SNARE proteins.
Conclusion: A Complex Dance of Ions and Molecules
The entry of calcium ions into the synaptic terminal is a fundamental event in neurotransmission. This process, far from being a simple on/off switch, is a highly regulated and finely tuned mechanism that relies on the intricate interplay of numerous proteins and ions. A thorough understanding of this intricate dance is essential for advancing our knowledge of brain function and developing effective treatments for neurological disorders. Future research continues to unveil the subtleties of calcium signaling in the presynaptic terminal, offering crucial insights into the complexity and elegance of neuronal communication. Further investigation into the various types of VGCCs, the specific roles of calcium-binding proteins, and the intricate dynamics of vesicle fusion and recycling promises to yield even greater comprehension of this critical process in the years to come. The continuous exploration of these mechanisms will undoubtedly deepen our understanding of the brain's complex machinery and pave the way for novel therapeutic strategies for a wide array of neurological conditions.
Latest Posts
Latest Posts
-
The Term Flattened Management Hierarchies Refers To
Mar 26, 2025
-
Which Of The Following Statements Are Correct
Mar 26, 2025
-
What Is Shown In The Image
Mar 26, 2025
-
Draw The Product S Of The Following Reactions
Mar 26, 2025
-
Which Of The Following Is Not A Property Of Water
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about When Calcium Ions Enter The Synaptic Terminal . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
