Draw The Product S Of The Following Reactions
Holbox
Mar 26, 2025 · 6 min read
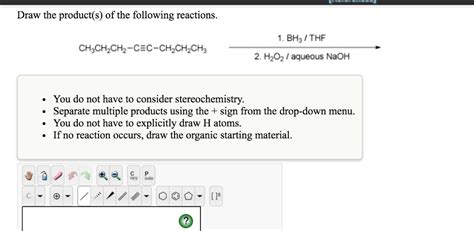
Table of Contents
- Draw The Product S Of The Following Reactions
- Table of Contents
- Predicting the Products of Chemical Reactions: A Comprehensive Guide
- Understanding Reaction Types: A Foundation for Prediction
- Advanced Considerations: Factors Influencing Reaction Outcomes
- Practical Strategies for Predicting Products
- Conclusion: Mastering the Art of Prediction
- Latest Posts
- Latest Posts
- Related Post
Predicting the Products of Chemical Reactions: A Comprehensive Guide
Predicting the products of chemical reactions is a fundamental skill in chemistry. It requires a strong understanding of reaction mechanisms, functional groups, and the principles of thermodynamics and kinetics. This comprehensive guide will explore various reaction types, providing strategies and examples to help you accurately predict the outcomes of chemical transformations. We’ll delve into several common reaction types, highlighting key considerations and potential pitfalls.
Understanding Reaction Types: A Foundation for Prediction
Before predicting products, it’s crucial to identify the type of reaction occurring. Common reaction types include:
1. Acid-Base Reactions: These reactions involve the transfer of a proton (H⁺) from an acid to a base. The strength of the acid and base dictates the equilibrium position. Strong acids react completely with strong bases, while weak acids and bases reach an equilibrium.
-
Predicting Products: Identify the acid and base. The acid will donate a proton to the base, forming the conjugate base of the acid and the conjugate acid of the base.
-
Example: HCl (acid) + NaOH (base) → NaCl (salt) + H₂O (water)
2. Redox Reactions (Oxidation-Reduction Reactions): These reactions involve the transfer of electrons. One species is oxidized (loses electrons), while another is reduced (gains electrons). Identifying the oxidizing and reducing agents is key.
-
Predicting Products: Determine the oxidation states of all atoms. Identify the species being oxidized and reduced. Balance the electrons transferred. The oxidized species will increase in oxidation state, and the reduced species will decrease.
-
Example: Zn (s) + Cu²⁺(aq) → Zn²⁺(aq) + Cu (s) (Zinc is oxidized, copper is reduced)
3. Precipitation Reactions: These reactions occur when two aqueous solutions containing soluble salts are mixed, resulting in the formation of an insoluble solid (precipitate). Solubility rules are crucial for predicting the formation of a precipitate.
-
Predicting Products: Identify the possible cation-anion combinations. Consult solubility rules to determine which combinations are insoluble. The insoluble combination forms the precipitate.
-
Example: AgNO₃(aq) + NaCl(aq) → AgCl(s) + NaNO₃(aq) (Silver chloride is the precipitate)
4. Combustion Reactions: These reactions involve the rapid reaction of a substance with oxygen, usually producing heat and light. Complete combustion of hydrocarbons produces carbon dioxide and water. Incomplete combustion may produce carbon monoxide and/or soot.
-
Predicting Products: For complete combustion of a hydrocarbon (CxHy), the products are CO₂ and H₂O. Balance the equation accordingly.
-
Example: CH₄ + 2O₂ → CO₂ + 2H₂O
5. Single Displacement Reactions: These involve one element replacing another in a compound. The reactivity series helps determine whether a reaction will occur. A more reactive element will displace a less reactive one.
-
Predicting Products: Consult the reactivity series. The more reactive element will replace the less reactive element in the compound.
-
Example: Fe (s) + CuSO₄(aq) → FeSO₄(aq) + Cu (s) (Iron is more reactive than copper)
6. Double Displacement Reactions: These involve the exchange of ions between two compounds. Often, these reactions result in the formation of a precipitate, water, or a gas.
-
Predicting Products: Identify the cations and anions in each reactant. Exchange the cations and anions to form new compounds. Consider solubility rules and acid-base reactions to determine the final products.
-
Example: BaCl₂(aq) + Na₂SO₄(aq) → BaSO₄(s) + 2NaCl(aq) (Barium sulfate is the precipitate)
7. Addition Reactions: These reactions involve adding atoms or groups of atoms to a molecule, typically an unsaturated compound (containing double or triple bonds).
-
Predicting Products: Identify the unsaturated bond. The atoms or groups being added will become bonded to the carbons involved in the double or triple bond. Consider Markovnikov's rule for addition reactions to unsymmetrical alkenes.
-
Example: CH₂=CH₂ + H₂ → CH₃-CH₃ (Hydrogenation of ethene)
8. Elimination Reactions: These reactions involve the removal of atoms or groups of atoms from a molecule, often resulting in the formation of a double or triple bond.
-
Predicting Products: Identify the atoms or groups being removed. The removal will result in the formation of a double or triple bond, and the removed atoms/groups will form a smaller molecule (e.g., water or HCl). Consider Zaitsev's rule for elimination reactions.
-
Example: CH₃-CH₂-OH → CH₂=CH₂ + H₂O (Dehydration of ethanol)
9. Substitution Reactions: These reactions involve the replacement of one atom or group of atoms with another. Nucleophilic substitution and electrophilic substitution are common types.
-
Predicting Products: Identify the leaving group and the incoming group. The leaving group will be replaced by the incoming group. Consider the reaction mechanism (SN1, SN2, etc.) for nucleophilic substitutions.
-
Example: CH₃-Br + OH⁻ → CH₃-OH + Br⁻ (Nucleophilic substitution)
Advanced Considerations: Factors Influencing Reaction Outcomes
Several factors beyond the basic reaction type can influence the products formed:
-
Reaction Conditions: Temperature, pressure, solvent, and the presence of catalysts significantly impact reaction pathways and product selectivity. Higher temperatures often favor faster reactions but can also lead to unwanted side reactions.
-
Stereochemistry: The three-dimensional arrangement of atoms in molecules can influence the products formed, particularly in addition and substitution reactions. Understanding stereochemistry is crucial for predicting the exact isomeric products.
-
Equilibrium Considerations: Many reactions are reversible, reaching an equilibrium between reactants and products. The position of equilibrium determines the relative amounts of reactants and products at equilibrium. Le Chatelier's principle helps predict the effect of changes in conditions on the equilibrium position.
-
Kinetic vs. Thermodynamic Control: Some reactions can lead to different products depending on whether the reaction is kinetically or thermodynamically controlled. Kinetic control favors the faster reaction, while thermodynamic control favors the more stable product.
-
Side Reactions: Unwanted side reactions can compete with the main reaction, leading to the formation of byproducts. Understanding potential side reactions is crucial for optimizing reaction conditions and improving product yields.
Practical Strategies for Predicting Products
To successfully predict the products of chemical reactions:
-
Identify the reaction type: Carefully analyze the reactants and their functional groups to determine the type of reaction that will likely occur.
-
Consider reaction mechanisms: Understanding the step-by-step process of a reaction provides insights into the formation of intermediates and final products.
-
Apply relevant rules and principles: Use solubility rules for precipitation reactions, oxidation state rules for redox reactions, and Markovnikov's/Zaitsev's rules for addition/elimination reactions.
-
Draw reaction mechanisms: Drawing out the reaction mechanism helps visualize the process and predict the structure of the products.
-
Balance the equation: Ensure that the number of atoms of each element is the same on both sides of the equation.
-
Consider reaction conditions: Account for the effects of temperature, pressure, solvent, and catalysts on the reaction pathway and product distribution.
-
Analyze potential side reactions: Be aware of possible side reactions that might compete with the desired reaction.
Conclusion: Mastering the Art of Prediction
Predicting the products of chemical reactions is a complex but rewarding skill. By mastering the various reaction types, applying relevant principles, and considering the influence of reaction conditions, you can significantly improve your ability to anticipate reaction outcomes. Consistent practice and a deep understanding of fundamental chemical concepts are essential for success in this area. Remember to always consult reliable resources and utilize systematic approaches to enhance your predictive capabilities. This skill is not only crucial for academic success but also forms the foundation of practical applications in various fields, including pharmaceuticals, materials science, and environmental chemistry. The more you practice, the more intuitive this process will become.
Latest Posts
Latest Posts
-
The Largest Expense For Most Airlines Is
Mar 29, 2025
-
Formal Education As An Approach To Employee Development Includes
Mar 29, 2025
-
Vail Company Recorded The Following Transactions During November
Mar 29, 2025
-
What Coversheet Is Attached To Help Protect A Secret Document
Mar 29, 2025
-
Elimination Reactions Are Favored Over Nucleophilic Substitution Reactions
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Draw The Product S Of The Following Reactions . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
