What Type Of Bonds Link Individual Amino Acids Together
Holbox
Mar 30, 2025 · 7 min read
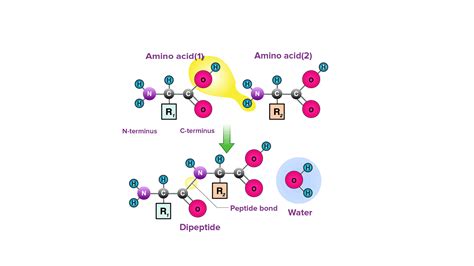
Table of Contents
- What Type Of Bonds Link Individual Amino Acids Together
- Table of Contents
- What Type of Bonds Link Individual Amino Acids Together?
- The Chemistry of Peptide Bond Formation
- Understanding the Peptide Bond's Characteristics
- The Role of Peptide Bonds in Protein Structure
- Primary Structure: The Linear Sequence
- Secondary Structure: Alpha-Helices and Beta-Sheets
- Tertiary Structure: Three-Dimensional Folding
- Quaternary Structure: Multi-Subunit Proteins
- Peptide Bond Hydrolysis: Breaking the Bonds
- Significance of Peptide Bonds in Biological Systems
- Beyond Peptide Bonds: Other Important Links in Proteins
- Conclusion: The Peptide Bond – A Cornerstone of Life
- Latest Posts
- Latest Posts
- Related Post
What Type of Bonds Link Individual Amino Acids Together?
Peptide bonds are the fundamental links connecting individual amino acids to form polypeptide chains, the building blocks of proteins. Understanding the nature of these bonds is crucial to comprehending protein structure, function, and the intricacies of biological processes. This article delves deep into the chemistry of peptide bonds, exploring their formation, characteristics, and significance in the broader context of biochemistry.
The Chemistry of Peptide Bond Formation
Amino acids, the monomers of proteins, possess a common structural feature: a central carbon atom (the α-carbon) bonded to an amino group (-NH₂), a carboxyl group (-COOH), a hydrogen atom (-H), and a variable side chain (R-group). This R-group distinguishes one amino acid from another, imparting unique chemical properties that contribute to the diverse functions of proteins.
The formation of a peptide bond is a dehydration reaction, also known as a condensation reaction. This process involves the removal of a water molecule (H₂O) when the carboxyl group of one amino acid reacts with the amino group of another. Specifically:
- The carboxyl group (-COOH) of one amino acid loses a hydroxyl group (-OH).
- The amino group (-NH₂) of the other amino acid loses a hydrogen atom (-H).
- The remaining carbon atom (C=O) from the carboxyl group and the nitrogen atom (N-H) from the amino group form a covalent bond, resulting in the peptide bond (-CO-NH-).
This reaction is energetically unfavorable under standard cellular conditions. Therefore, it requires the input of energy, usually provided by ATP (adenosine triphosphate) through the involvement of enzymes such as aminoacyl-tRNA synthetases during protein synthesis (translation) or through enzymatic processes in other biosynthetic pathways.
Understanding the Peptide Bond's Characteristics
The peptide bond, despite being a covalent bond, possesses some unique characteristics that influence protein structure and function:
-
Partial Double Bond Character: The peptide bond exhibits resonance. This means that the electrons involved in the bond are delocalized, creating a partial double bond character between the carbon and nitrogen atoms. This partial double bond restricts rotation around the C-N bond, influencing the overall conformation of the polypeptide chain. This rigidity is critical in determining the protein's secondary structure (alpha-helices and beta-sheets).
-
Planarity: Due to the partial double bond character, the atoms involved in the peptide bond (C=O, N-H, and the α-carbons) lie in a relatively planar configuration. This planarity contributes to the overall shape of the protein molecule.
-
Polarity: The peptide bond is polar due to the electronegativity difference between the oxygen (O) and nitrogen (N) atoms. This polarity influences the interaction of the polypeptide chain with water molecules and other polar molecules within the cellular environment. The oxygen atom carries a partial negative charge (δ-), while the nitrogen carries a partial positive charge (δ+). This dipole contributes to hydrogen bonding, a crucial force in protein folding and stability.
-
Trans Configuration: In most peptide bonds, the amino acid side chains (R-groups) are oriented on opposite sides of the peptide bond (trans configuration). This is energetically more favorable than the cis configuration, where the R-groups are on the same side. However, exceptions exist, particularly in proline residues where the cis configuration can be observed.
The Role of Peptide Bonds in Protein Structure
The peptide bond's properties are directly related to the various levels of protein structure:
Primary Structure: The Linear Sequence
The primary structure of a protein refers to the linear sequence of amino acids linked together by peptide bonds. This sequence is dictated by the genetic code and determines all higher levels of protein structure. Any change in this sequence (e.g., a single amino acid substitution) can significantly affect the protein's function, as observed in genetic diseases like sickle cell anemia.
Secondary Structure: Alpha-Helices and Beta-Sheets
The peptide bond's partial double bond character and planarity significantly influence the formation of secondary structures. Hydrogen bonds between the carbonyl oxygen (C=O) of one peptide bond and the amide hydrogen (N-H) of another peptide bond four residues down the chain stabilize alpha-helices. In beta-sheets, hydrogen bonds form between adjacent polypeptide chains or between different segments of the same polypeptide chain, creating a pleated sheet-like structure.
Tertiary Structure: Three-Dimensional Folding
The three-dimensional structure of a protein is largely determined by the interactions between its amino acid side chains (R-groups). These interactions include:
- Hydrophobic interactions: Nonpolar side chains cluster together in the protein's interior, away from the aqueous environment.
- Hydrophilic interactions: Polar side chains interact with water molecules on the protein's surface.
- Hydrogen bonds: Form between polar side chains and the backbone peptide bonds.
- Ionic bonds (salt bridges): Form between oppositely charged side chains.
- Disulfide bonds: Covalent bonds that form between cysteine residues, contributing significantly to protein stability.
The peptide backbone, with its sequence of peptide bonds, provides the scaffold upon which these interactions occur, leading to the unique three-dimensional folding of the protein.
Quaternary Structure: Multi-Subunit Proteins
Some proteins consist of multiple polypeptide chains (subunits) that associate to form a functional protein complex. The interactions between these subunits, like those in tertiary structure, are stabilized by various forces, including hydrophobic interactions, hydrogen bonds, ionic bonds, and sometimes disulfide bonds. The peptide bonds within each subunit define the individual polypeptide chain's structure and contribute to the overall quaternary structure's stability and functionality.
Peptide Bond Hydrolysis: Breaking the Bonds
Peptide bonds are not immutable. They can be broken through a process called hydrolysis, which is the reverse of the dehydration reaction forming the bond. This process requires the addition of a water molecule and usually involves enzymes, such as proteases or peptidases. Hydrolysis of peptide bonds plays essential roles in:
- Protein degradation: Cells constantly degrade and recycle proteins, breaking down old or damaged proteins into individual amino acids.
- Digestion: The digestive system breaks down dietary proteins into amino acids through enzymatic hydrolysis of peptide bonds.
- Protein processing: Some proteins are synthesized as larger precursors (proproteins) that require cleavage of specific peptide bonds to become active.
Significance of Peptide Bonds in Biological Systems
The peptide bond's fundamental role in linking amino acids is paramount to life. The properties of this bond dictate the structure and function of proteins, which are involved in virtually every biological process:
- Enzymes: Catalyze biochemical reactions.
- Structural proteins: Provide support and shape (e.g., collagen, keratin).
- Transport proteins: Carry molecules across membranes (e.g., hemoglobin).
- Hormones: Regulate physiological processes (e.g., insulin, glucagon).
- Antibodies: Defend against pathogens.
- Receptors: Bind to signaling molecules.
- Motor proteins: Generate movement (e.g., myosin, kinesin).
Beyond Peptide Bonds: Other Important Links in Proteins
While peptide bonds are the primary links connecting amino acids in a polypeptide chain, other types of chemical interactions contribute to the overall structure and stability of proteins:
- Disulfide bonds: Covalent bonds between cysteine residues, crucial for stabilizing tertiary and quaternary structures.
- Hydrogen bonds: Weak bonds involving hydrogen atoms and electronegative atoms (oxygen or nitrogen), essential for secondary, tertiary, and quaternary structures.
- Ionic bonds (salt bridges): Electrostatic attractions between oppositely charged side chains.
- Hydrophobic interactions: Clustering of nonpolar side chains within the protein's core, driven by the tendency of water to exclude nonpolar molecules.
- Van der Waals forces: Weak attractive forces between atoms in close proximity.
Conclusion: The Peptide Bond – A Cornerstone of Life
The peptide bond, a seemingly simple covalent link, is a cornerstone of life's intricate molecular machinery. Its unique characteristics—the partial double bond character, planarity, polarity, and susceptibility to hydrolysis—underpin the diverse structures and functions of proteins. Understanding the chemistry of peptide bonds is fundamental to comprehending protein structure, function, and the vast array of biological processes they orchestrate. Further research continues to unveil the subtleties of peptide bond behavior and its implications for protein folding, stability, and disease, underscoring its enduring significance in biological systems. From the simplest bacteria to the most complex organisms, the peptide bond serves as a vital molecular connector, building the intricate tapestry of life.
Latest Posts
Latest Posts
-
Which Of The Following Occurs During Data Cleansing
Apr 02, 2025
-
What Was Your First Choice Using The Winre Option Menu
Apr 02, 2025
-
Correctly Label The Following External Anatomy Of The Posterior Heart
Apr 02, 2025
-
Performance Feedback Is Most Effective When It
Apr 02, 2025
-
The Effective Management Of Accounts Receivable Requires Financial Managers To
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Type Of Bonds Link Individual Amino Acids Together . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
