What Is The Predicted Product Of The Reaction Shown
Holbox
Mar 10, 2025 · 6 min read
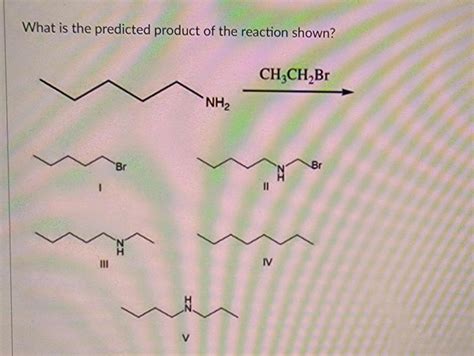
Table of Contents
What is the Predicted Product of the Reaction Shown? A Comprehensive Guide to Predicting Organic Reaction Outcomes
Predicting the outcome of a chemical reaction is a cornerstone of organic chemistry. It's a skill honed through understanding reaction mechanisms, functional group transformations, and the interplay of various reaction conditions. This article delves into the process of predicting reaction products, offering a structured approach and numerous examples to solidify your understanding. We'll explore various reaction types, including substitution, elimination, addition, and redox reactions, providing you with the tools to confidently predict the major product(s) formed in a given reaction.
Understanding Reaction Mechanisms: The Key to Prediction
Before jumping into specific reactions, let's establish a firm foundation: reaction mechanisms. A reaction mechanism is a detailed step-by-step description of how a reaction occurs at the molecular level. Understanding the mechanism allows you to predict not only the product but also the stereochemistry (spatial arrangement of atoms) and regiochemistry (position of substituents on a molecule) of the product.
Key Concepts in Reaction Mechanisms:
- Nucleophiles (Nu⁻): Electron-rich species that donate electrons to electrophilic centers. Examples include hydroxide ions (OH⁻), alkoxide ions (RO⁻), and amines (RNH₂).
- Electrophiles (E⁺): Electron-deficient species that accept electrons from nucleophiles. Examples include carbocations, carbonyl carbons, and alkyl halides.
- Leaving Groups (LG): Atoms or groups that depart from a molecule, taking a pair of electrons with them. Common leaving groups include halides (Cl⁻, Br⁻, I⁻), water (H₂O), and tosylates (OTs).
- Intermediates: Transient species formed during the reaction that are neither reactants nor products. Common intermediates include carbocations and carbanions.
- Transition States: High-energy states representing the maximum energy point along the reaction coordinate. These are short-lived and not isolable.
Major Reaction Types and Product Prediction
Now, let's explore some of the most common reaction types in organic chemistry and how to predict their products.
1. Nucleophilic Substitution Reactions (SN1 and SN2)
These reactions involve the replacement of a leaving group (LG) by a nucleophile (Nu⁻). There are two main mechanisms:
-
SN2 (Bimolecular Nucleophilic Substitution): A one-step mechanism where the nucleophile attacks the carbon atom bearing the leaving group from the backside, simultaneously displacing the leaving group. This leads to inversion of configuration at the chiral center.
Predicting the Product: Identify the nucleophile and the leaving group. The nucleophile replaces the leaving group, resulting in a new bond between the nucleophile and the carbon atom. In a chiral molecule, the configuration inverts.
-
SN1 (Unimolecular Nucleophilic Substitution): A two-step mechanism involving the formation of a carbocation intermediate. The leaving group departs first, forming a carbocation. The nucleophile then attacks the carbocation. This often leads to a racemic mixture (equal amounts of both enantiomers) if the carbocation is planar.
Predicting the Product: Identify the leaving group. The leaving group departs, forming a carbocation. The nucleophile attacks the carbocation, forming a new bond. If the starting material is chiral, the product will likely be a racemic mixture unless there are steric factors influencing the nucleophilic attack.
2. Elimination Reactions (E1 and E2)
Elimination reactions involve the removal of two atoms or groups from adjacent carbon atoms, resulting in the formation of a double bond (alkene).
-
E2 (Bimolecular Elimination): A one-step mechanism where the base abstracts a proton from a carbon atom adjacent to the carbon bearing the leaving group, while the leaving group departs simultaneously. The stereochemistry is important; anti-periplanar geometry is favored.
Predicting the Product: Identify the base and the leaving group. Determine the most substituted alkene (Zaitsev's rule generally predicts the major product). Consider the stereochemistry of the starting material; anti-periplanar geometry is necessary for efficient E2 elimination.
-
E1 (Unimolecular Elimination): A two-step mechanism involving the formation of a carbocation intermediate. The leaving group departs first, forming a carbocation, and then a base abstracts a proton from an adjacent carbon atom, forming the alkene.
Predicting the Product: Identify the leaving group. The leaving group departs, forming a carbocation. A base abstracts a proton from an adjacent carbon atom, forming the alkene. The most substituted alkene (Zaitsev's rule) is usually the major product.
3. Addition Reactions
Addition reactions involve the addition of atoms or groups across a multiple bond (double or triple bond). Common addition reactions include:
-
Electrophilic Addition: Electrophiles add to the multiple bond, forming a carbocation intermediate which is then attacked by a nucleophile. Markovnikov's rule often applies, predicting that the electrophile adds to the more substituted carbon atom.
Predicting the Product: Identify the electrophile and the nucleophile. The electrophile adds to the more substituted carbon (Markovnikov's rule), and the nucleophile adds to the less substituted carbon.
-
Free Radical Addition: Involves the addition of free radicals to multiple bonds, often initiated by light or heat. The regioselectivity may differ from electrophilic addition.
Predicting the Product: Consider the stability of the intermediate radicals. The more substituted radical is generally more stable.
4. Redox Reactions
Redox reactions involve the transfer of electrons between reactants. Common redox reactions in organic chemistry include oxidation and reduction of alcohols, aldehydes, ketones, and carboxylic acids.
**Predicting the Product:** Identify the oxidizing or reducing agent. Consider the functional group being oxidized or reduced and the common oxidation states.
Factors Affecting Product Prediction
Several factors can influence the outcome of a reaction, and accurate product prediction requires consideration of these variables:
- Temperature: Higher temperatures often favor elimination reactions over substitution reactions.
- Solvent: Polar protic solvents favor SN1 and E1 reactions, while polar aprotic solvents favor SN2 reactions.
- Strength of the Nucleophile/Base: Strong nucleophiles/bases favor SN2 and E2 reactions, respectively.
- Steric Hindrance: Bulky substituents can hinder reactions, affecting the rate and regioselectivity.
Advanced Techniques for Predicting Reaction Outcomes
- Computational Chemistry: Molecular modeling and computational methods can provide insights into reaction mechanisms and energy profiles, allowing for more accurate product prediction.
- Spectroscopic Techniques: NMR, IR, and mass spectrometry can provide experimental data that confirms or refutes predicted product structures.
Conclusion: A Continuous Learning Process
Predicting the products of organic reactions is a complex but rewarding skill. While mastering this skill requires a deep understanding of reaction mechanisms and influencing factors, the systematic approach outlined above offers a powerful framework. This is a continuous learning process, with practice and experience gradually refining your predictive abilities. Remember to carefully analyze the reactants, reaction conditions, and potential intermediates to make informed predictions, and always consult available resources and experimental data to validate your conclusions. By diligently applying the principles discussed here, you will significantly improve your ability to anticipate the outcomes of a wide range of organic reactions.
Latest Posts
Latest Posts
-
This Term Identifies Any Network Node
Mar 10, 2025
-
The Tax In Cell F2 Must Be Applied
Mar 10, 2025
-
A Planned Evacuation Drill Should Occur
Mar 10, 2025
-
Two Fishermen Are In A 4 Meter Boat
Mar 10, 2025
-
Julia Is A Customer Sitting At Your Bar
Mar 10, 2025
Related Post
Thank you for visiting our website which covers about What Is The Predicted Product Of The Reaction Shown . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
