What Is The Most Likely Product Of The Following Reaction
Holbox
Mar 24, 2025 · 5 min read
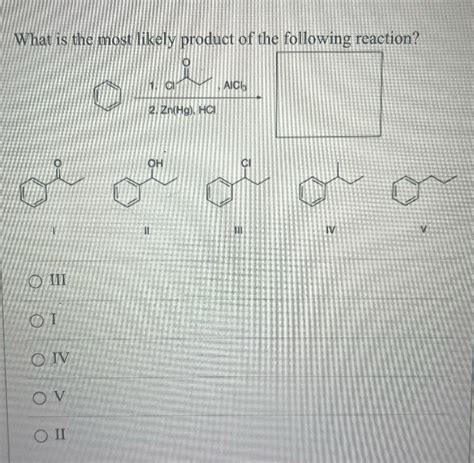
Table of Contents
- What Is The Most Likely Product Of The Following Reaction
- Table of Contents
- Predicting the Major Product: A Deep Dive into Organic Reaction Mechanisms
- Understanding Reaction Mechanisms: The Key to Prediction
- Factors Determining Product Distribution: Regioselectivity, Stereoselectivity, and Chemoselectivity
- Advanced Considerations in Product Prediction
- Practical Approach to Predicting the Major Product
- Conclusion: Mastering the Art of Prediction
- Latest Posts
- Latest Posts
- Related Post
Predicting the Major Product: A Deep Dive into Organic Reaction Mechanisms
Predicting the major product of a chemical reaction is a cornerstone of organic chemistry. It requires a deep understanding of reaction mechanisms, including factors influencing reaction rates and selectivity. This article explores the strategies used to predict the most likely product, focusing on common reaction types and the nuances that determine product distribution. We'll delve into concepts like regioselectivity, stereoselectivity, and chemoselectivity, illustrating the principles with examples. While providing specific examples isn't possible without knowing the specific reaction in question, we will discuss the general approaches to effectively tackle such problems.
Understanding Reaction Mechanisms: The Key to Prediction
The foundation of predicting the major product lies in comprehending the reaction mechanism. A reaction mechanism is a step-by-step description of how a reaction proceeds, detailing the movement of electrons and the formation and breaking of bonds. Different reaction mechanisms favor different products. Understanding these pathways is paramount.
Key mechanistic concepts influencing product formation:
-
Nucleophilic attack: Nucleophiles, electron-rich species, attack electrophilic centers, electron-deficient regions. The nucleophile's strength and the electrophile's susceptibility heavily influence the product formed.
-
Electrophilic attack: Electrophiles, electron-deficient species, seek out nucleophilic sites. The electrophile's reactivity and the nucleophile's availability are crucial factors.
-
Carbocation rearrangements: Carbocations, positively charged carbon atoms, are highly reactive intermediates. They readily undergo rearrangements (hydride or alkyl shifts) to form more stable carbocations, leading to different products than initially expected.
-
SN1 vs. SN2 reactions: These nucleophilic substitution reactions differ significantly. SN1 reactions proceed through a carbocation intermediate, making them prone to rearrangements and less stereospecific. SN2 reactions are concerted, meaning the nucleophile attacks simultaneously as the leaving group departs, resulting in inversion of stereochemistry.
-
E1 vs. E2 eliminations: These elimination reactions also differ significantly in their mechanisms. E1 reactions proceed through a carbocation intermediate, while E2 reactions are concerted. The reaction conditions (strong base for E2, weak base/heat for E1) play a vital role in determining which pathway is favoured.
-
Addition reactions: These reactions involve the addition of reactants across a double or triple bond. Markovnikov's rule and anti-Markovnikov addition are important considerations in predicting the regioselectivity of these reactions.
-
Substitution reactions: These reactions involve the replacement of one atom or group by another. The nature of the leaving group, the nucleophile, and the substrate will influence the course of the reaction.
Factors Determining Product Distribution: Regioselectivity, Stereoselectivity, and Chemoselectivity
Several factors govern the distribution of products in a reaction. Understanding these concepts is essential for accurate predictions:
1. Regioselectivity: This refers to the preference for reaction at one particular position over another in a molecule with multiple reactive sites. For example, in the addition of HX to an unsymmetrical alkene, Markovnikov's rule predicts the major product will be the one where the hydrogen atom adds to the carbon atom with more hydrogen atoms already attached.
2. Stereoselectivity: This refers to the preference for the formation of one stereoisomer over another. It's particularly relevant in reactions involving chiral centers. Factors influencing stereoselectivity include steric hindrance, approach of the reagent, and the reaction mechanism. For example, SN2 reactions are stereospecific, leading to inversion of configuration.
3. Chemoselectivity: This refers to the selective reaction of one functional group over another in a molecule containing multiple functional groups. The reactivity of each functional group and the reaction conditions dictate which group reacts preferentially. For example, a selective reduction can target a ketone group in the presence of an ester group.
Advanced Considerations in Product Prediction
Beyond the basic mechanistic concepts, several factors can influence the major product formed:
-
Steric effects: Bulky groups can hinder reaction pathways, influencing the regioselectivity and stereoselectivity of the reaction.
-
Electronic effects: Electron-donating or electron-withdrawing groups can affect the reactivity of different sites in a molecule.
-
Reaction conditions: Temperature, solvent, and the concentration of reactants can significantly influence the product distribution. Certain conditions might favor one pathway over another, even if both are thermodynamically possible.
-
Kinetic vs. Thermodynamic control: Sometimes, the major product depends on whether the reaction is under kinetic or thermodynamic control. Kinetic control favors the product formed faster, while thermodynamic control favors the most stable product.
Practical Approach to Predicting the Major Product
Predicting the major product requires a systematic approach:
-
Identify the functional groups: Determine the reactive sites in the molecule.
-
Determine the type of reaction: Is it a nucleophilic substitution, electrophilic addition, elimination, etc.?
-
Propose a mechanism: Draw out the step-by-step mechanism, showing the movement of electrons.
-
Consider regioselectivity, stereoselectivity, and chemoselectivity: Identify factors influencing the preferential formation of one product over another.
-
Account for steric and electronic effects: Assess how these factors influence the reaction pathway.
-
Analyze the reaction conditions: Determine if the conditions favor kinetic or thermodynamic control.
-
Evaluate the stability of intermediates and products: More stable intermediates and products are more likely to form.
-
Draw the major product: Based on your analysis, identify the most likely product formed.
-
Consider minor products: While predicting the major product is the primary goal, it's also important to consider the possibility of minor products forming via alternative pathways.
Conclusion: Mastering the Art of Prediction
Predicting the major product of a reaction is not a simple task; it demands a thorough understanding of organic chemistry principles. However, by systematically applying the concepts of reaction mechanisms, regioselectivity, stereoselectivity, chemoselectivity, and considering factors like sterics, electronics, and reaction conditions, one can significantly improve their ability to predict the most probable outcome of a reaction. Remember, practice is key. Working through numerous examples and applying these principles will build your proficiency and confidence in predicting the major product of a wide range of organic reactions. Continuously refining your understanding of reaction mechanisms and their nuances is an ongoing journey for any organic chemist.
Latest Posts
Latest Posts
-
Bonita Company Manufactures A Single Product
Mar 27, 2025
-
You Want To Print Each Slide On Its Own Page
Mar 27, 2025
-
Which Statement Regarding Roys Theory Of Nursing Needs Correction
Mar 27, 2025
-
Unlike Tort Law Contract Law States That
Mar 27, 2025
-
A Block Initially At Rest Is Given A Quick Push
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about What Is The Most Likely Product Of The Following Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
