What Is The Mass Of 3.81 Mol Of Ph3
Holbox
Mar 25, 2025 · 5 min read
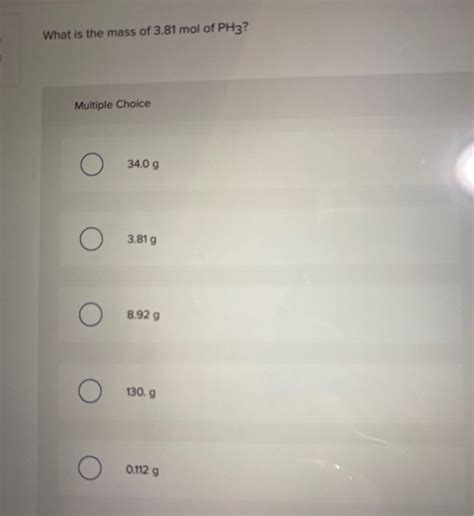
Table of Contents
- What Is The Mass Of 3.81 Mol Of Ph3
- Table of Contents
- What is the Mass of 3.81 mol of PH₃? A Deep Dive into Molar Mass and Stoichiometry
- Understanding Molar Mass: The Bridge Between Moles and Grams
- Calculating the Mass of 3.81 Moles of PH₃
- Significance of Molar Mass and its Applications
- 1. Stoichiometric Calculations:
- 2. Solution Preparation:
- 3. Analytical Chemistry:
- 4. Industrial Processes:
- 5. Environmental Science:
- Potential Sources of Error and Precision Considerations
- Expanding the Understanding: Beyond PH₃
- Conclusion: Molar Mass – A Cornerstone of Chemical Calculations
- Latest Posts
- Latest Posts
- Related Post
What is the Mass of 3.81 mol of PH₃? A Deep Dive into Molar Mass and Stoichiometry
Determining the mass of a given number of moles of a substance is a fundamental concept in chemistry, crucial for various applications from stoichiometric calculations to lab experiments. This article will thoroughly explore how to calculate the mass of 3.81 moles of phosphine (PH₃), providing a comprehensive understanding of the underlying principles and related concepts. We will also delve into the significance of molar mass, its calculation, and its application in solving similar problems.
Understanding Molar Mass: The Bridge Between Moles and Grams
Before we tackle the specific problem, let's solidify our understanding of molar mass. Molar mass is the mass of one mole of a substance. One mole is defined as 6.022 x 10²³ (Avogadro's number) of elementary entities, which could be atoms, molecules, ions, or other specified particles. The units of molar mass are typically grams per mole (g/mol).
Calculating Molar Mass:
The molar mass of a compound is calculated by adding the atomic masses of all the atoms present in its chemical formula. Atomic masses are typically found on the periodic table. For PH₃ (phosphine), we need the atomic masses of phosphorus (P) and hydrogen (H).
- Phosphorus (P): Approximately 30.97 g/mol
- Hydrogen (H): Approximately 1.01 g/mol
Therefore, the molar mass of PH₃ is:
(1 x 30.97 g/mol) + (3 x 1.01 g/mol) = 33.97 g/mol
This means that one mole of PH₃ weighs approximately 33.97 grams.
Calculating the Mass of 3.81 Moles of PH₃
Now that we know the molar mass of PH₃, we can easily calculate the mass of 3.81 moles. We'll use the following formula:
Mass (g) = Number of moles (mol) x Molar mass (g/mol)
Plugging in the values:
Mass (g) = 3.81 mol x 33.97 g/mol
Mass (g) ≈ 129.47 g
Therefore, the mass of 3.81 moles of PH₃ is approximately 129.47 grams.
Significance of Molar Mass and its Applications
The concept of molar mass is fundamental to many areas of chemistry and related fields. Here are some key applications:
1. Stoichiometric Calculations:
Molar mass is essential for performing stoichiometric calculations, which involve determining the amounts of reactants and products in a chemical reaction. It allows us to convert between mass and moles, enabling us to use balanced chemical equations to predict the quantities of substances involved.
2. Solution Preparation:
Molar mass is crucial in preparing solutions of a specific concentration. For example, to prepare a 1 M (molar) solution of PH₃, you would need to dissolve one mole (33.97 g) of PH₃ in enough solvent to make a 1-liter solution.
3. Analytical Chemistry:
Molar mass is used extensively in analytical chemistry techniques, including titration, gravimetric analysis, and spectrophotometry. These techniques often involve determining the amount of a substance present in a sample, and molar mass is a critical factor in converting the measured quantity to moles and then to mass.
4. Industrial Processes:
In industrial processes, accurate calculations of molar masses are essential for optimizing reaction yields, controlling product purity, and managing the efficient use of raw materials.
5. Environmental Science:
Molar mass plays a crucial role in understanding and quantifying the concentrations of pollutants and other substances in environmental samples (air, water, soil). Accurate calculations help assess environmental risks and develop effective remediation strategies.
Potential Sources of Error and Precision Considerations
While our calculation provides a good approximation, it's crucial to acknowledge potential sources of error and the importance of precision:
-
Atomic Mass Precision: The atomic masses used are average values based on the isotopic composition of the elements. Slight variations in isotopic abundance could lead to minor discrepancies in the calculated molar mass.
-
Significant Figures: The number of significant figures in the final answer should reflect the precision of the input values. In this case, using 3.81 moles and 33.97 g/mol suggests a reasonable level of precision, resulting in an answer of approximately 129.47 g. However, depending on the context and the precision required, it might be appropriate to round the final answer to fewer significant figures.
-
Experimental Error: In any real-world experiment involving measuring the mass of a substance, various sources of experimental error could affect the accuracy of the results. These could include weighing errors, impurities in the substance, and losses during the experimental process.
Expanding the Understanding: Beyond PH₃
The principles discussed here apply equally well to other compounds. To calculate the mass of a specific number of moles of any compound, you need only determine its molar mass using the periodic table and apply the same formula:
Mass (g) = Number of moles (mol) x Molar mass (g/mol)
For example, if you needed to find the mass of 2.5 moles of water (H₂O), you would first calculate the molar mass of H₂O:
(2 x 1.01 g/mol) + (1 x 16.00 g/mol) = 18.02 g/mol
Then, you would calculate the mass:
Mass (g) = 2.5 mol x 18.02 g/mol = 45.05 g
Conclusion: Molar Mass – A Cornerstone of Chemical Calculations
The calculation of the mass of 3.81 moles of PH₃, as we've shown, exemplifies the fundamental importance of molar mass in chemistry. Understanding how to calculate molar mass and how to use it in stoichiometric calculations is essential for success in various chemical disciplines and related fields. By grasping these concepts, you gain a powerful tool for understanding and manipulating chemical quantities, laying a solid foundation for more advanced chemical studies. Remember always to consider the precision of your measurements and the potential sources of error to obtain reliable results. This comprehensive approach to solving this seemingly simple problem showcases the rich underlying principles in chemistry and its practical applications.
Latest Posts
Latest Posts
-
Rn Targeted Medical Surgical Renal And Urinary Online Practice 2023
Mar 26, 2025
-
The Margin Of Safety Is The Excess Of
Mar 26, 2025
-
Food Preservation Does All The Following Except
Mar 26, 2025
-
Use The Graphs To Evaluate The Expressions Below
Mar 26, 2025
-
Which Of These Gametes Contains One Or More Recombinant Chromosomes
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Is The Mass Of 3.81 Mol Of Ph3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
