What Is The Iupac Name Of The Following Compound
Holbox
Mar 12, 2025 · 5 min read
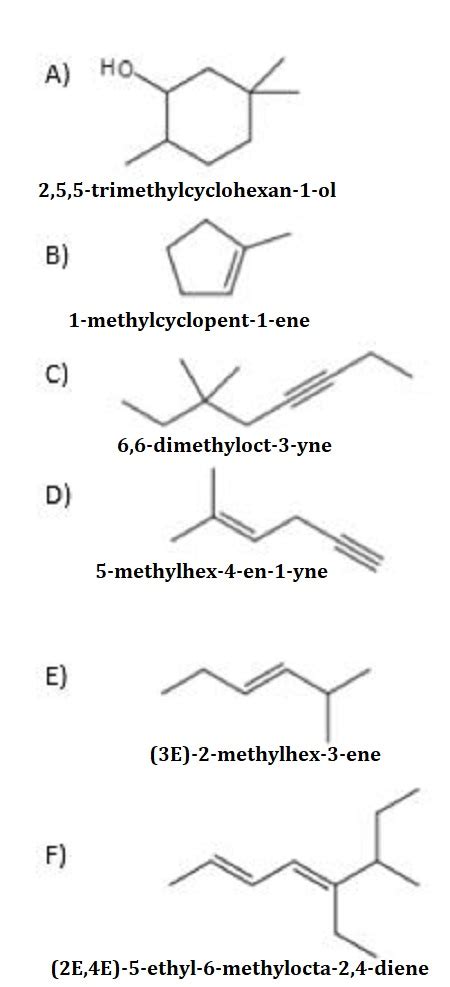
Table of Contents
Decoding Chemical Structures: A Deep Dive into IUPAC Nomenclature
Naming chemical compounds might seem like a daunting task, especially when faced with complex structures. However, the International Union of Pure and Applied Chemistry (IUPAC) has established a systematic nomenclature system to ensure clarity and unambiguous communication within the scientific community. This system allows chemists worldwide to understand exactly which molecule is being discussed, regardless of language or regional differences. This article will delve into the principles of IUPAC nomenclature, providing a comprehensive guide to naming organic compounds, with a focus on understanding the logic and methodology behind the process. We'll tackle various functional groups, branching, and complexities to equip you with the skills to name even the most intricate molecules.
Before we embark on this journey, let's emphasize that without a specific chemical structure, providing the IUPAC name is impossible. The question "What is the IUPAC name of the following compound?" requires the following compound to be presented. Therefore, this article will provide a framework for naming various types of organic compounds, illustrating the process with examples. You can then apply this framework to any given structure.
Understanding the Fundamentals of IUPAC Nomenclature
The IUPAC system follows a set of rules and priorities to systematically name organic compounds. The core principles include:
- Identifying the Parent Chain: This is the longest continuous carbon chain in the molecule.
- Identifying Substituents: These are atoms or groups of atoms attached to the parent chain.
- Numbering the Carbon Atoms: The parent chain is numbered to give the substituents the lowest possible numbers.
- Naming the Substituents: Each substituent is named individually, and its position on the parent chain is indicated by a number.
- Alphabetizing the Substituents: The substituents are listed alphabetically, ignoring prefixes like di- or tri- (except for iso, sec, and tert).
- Combining the Information: The final name is constructed by combining the names and positions of the substituents with the name of the parent chain.
Alkanes: The Foundation of Organic Nomenclature
Alkanes are hydrocarbons containing only single bonds. Their names form the basis for naming many other organic compounds. The first ten alkanes are: methane (CH₄), ethane (C₂H₆), propane (C₃H₈), butane (C₄H₁₀), pentane (C₅H₁₂), hexane (C₆H₁₄), heptane (C₇H₁₆), octane (C₈H₁₈), nonane (C₉H₂₀), and decane (C₁₀H₂₂). The names follow a systematic pattern, with the suffix "-ane" indicating an alkane.
Example: Consider a branched alkane with four carbons:
CH₃
|
CH₃-CH-CH₃
- Parent Chain: The longest continuous carbon chain has three carbons, making it propane.
- Substituent: A methyl group (CH₃) is attached to the central carbon.
- Numbering: Number the propane chain such that the methyl group gets the lowest number (2).
- Name: 2-methylpropane
Alkenes and Alkynes: Incorporating Double and Triple Bonds
Alkenes contain at least one carbon-carbon double bond, while alkynes contain at least one carbon-carbon triple bond. The suffix "-ene" is used for alkenes, and "-yne" for alkynes. The position of the double or triple bond is indicated by a number.
Example (Alkene):
CH₂=CH-CH₂-CH₃
This is 1-butene. The double bond is between carbons 1 and 2.
Example (Alkyne):
CH≡C-CH₂-CH₃
This is 1-butyne. The triple bond is between carbons 1 and 2.
Alcohols, Aldehydes, Ketones, and Carboxylic Acids: Functional Group Considerations
Functional groups are specific atoms or groups of atoms within a molecule that impart characteristic chemical properties. The IUPAC system incorporates the names of these functional groups into the compound's name, often modifying the suffix or adding prefixes.
-
Alcohols (-OH): The suffix "-ol" is added to the parent alkane name, and the position of the hydroxyl group (-OH) is indicated by a number. For example, CH₃CH₂OH is ethanol.
-
Aldehydes (-CHO): The suffix "-al" is used. The aldehyde group is always at the end of the chain, so numbering is not necessary. For example, CH₃CHO is ethanal.
-
Ketones (C=O): The suffix "-one" is used, and the position of the carbonyl group (C=O) is indicated by a number. For example, CH₃COCH₃ is propanone (also known as acetone).
-
Carboxylic Acids (-COOH): The suffix "-oic acid" is used. The carboxyl group is always at the end of the chain. For example, CH₃COOH is ethanoic acid (also known as acetic acid).
Cyclic Compounds: Navigating Rings
Cyclic compounds contain rings of carbon atoms. The prefix "cyclo-" is added to the parent alkane name to indicate a cyclic structure. For example, a six-membered carbon ring is called cyclohexane. Substituents on the ring are numbered to give the lowest possible numbers.
Example:
CH₃
|
CH₃-CH-CH₂
\ /
C
/ \
CH₂-CH₂
This is 1-methyl-2-ethylcyclopentane
More Complex Structures: Handling Multiple Substituents and Complex Functional Groups
As the complexity of the molecules increases, so does the challenge of naming them. However, the basic principles of the IUPAC system still apply. Multiple substituents are named individually and listed alphabetically, with their positions indicated by numbers. If there are multiple identical substituents, prefixes like di, tri, tetra, etc., are used. When multiple functional groups are present, a hierarchy of priority dictates which group determines the base name and suffix. Carboxylic acids have the highest priority, followed by aldehydes, ketones, alcohols, and so on.
Example (Multiple Substituents):
Consider a molecule with both methyl and ethyl substituents:
CH₃ CH₂CH₃
\ /
C-C-C
/ \
CH₂CH₃ CH₃
This is 2,3-diethyl-2,3-dimethylbutane
Systematic Approach and Practice: Mastering IUPAC Nomenclature
Mastering IUPAC nomenclature requires a systematic approach and consistent practice. Begin with simpler structures and gradually increase the complexity. Familiarize yourself with the different functional groups and their associated suffixes and prefixes. Use online resources and textbooks to practice naming compounds and verify your answers. Remember, consistent practice is key to developing proficiency in this essential skill for any aspiring chemist.
This in-depth guide provides a comprehensive overview of IUPAC nomenclature. However, due to the vastness and complexity of organic chemistry, this explanation only touches upon the fundamentals. Remember that specific structural information is crucial for accurate naming. By understanding these principles and practicing regularly, you will significantly enhance your ability to name and understand the vast array of organic compounds. This understanding is essential not only for academic pursuits but also for various applications in research, industry, and beyond. The ability to accurately and efficiently name chemical compounds is a fundamental skill in chemistry, fostering clear communication and collaboration within the scientific community.
Latest Posts
Latest Posts
-
The New Product Process Stage Of Screening And Evaluation Involves
Mar 14, 2025
-
Consider A Binomial Experiment With And
Mar 14, 2025
-
Independent Auditors Express An Opinion On The
Mar 14, 2025
-
A Focus On Customer Orientation Leads To Improved
Mar 14, 2025
-
Data Table 1 Lab Safety Equipment Alternatives
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about What Is The Iupac Name Of The Following Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
