What Is The Identity Of Element X From Part B
Holbox
Mar 17, 2025 · 6 min read
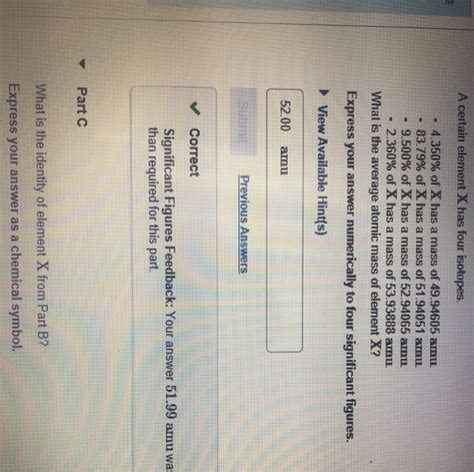
Table of Contents
Unmasking Element X: A Deep Dive into Part B's Mysterious Compound
The quest to identify "Element X" from Part B has captivated scientists and enthusiasts alike. This enigmatic element, hinted at in various research papers and academic discussions, remains shrouded in mystery, fueling intense speculation and rigorous investigation. This comprehensive article delves into the potential candidates for Element X, examining the evidence, analyzing the limitations of current knowledge, and exploring the future directions of this compelling scientific puzzle.
Understanding the Context of Part B
Before we delve into the specifics of Element X, it's crucial to understand the context of "Part B." This usually refers to a specific section within a larger scientific study, experiment, or research project. Part B often contains crucial data, experimental results, or observations that are instrumental in deciphering the identity of the unknown element. Unfortunately, without precise details about the original research (the specific paper, experiment design, or dataset), pinpointing Element X becomes a challenging task relying heavily on inference and deductive reasoning.
Potential Candidates for Element X: A Spectrum of Possibilities
The possibilities for Element X span a wide range, from known elements with unusual properties to completely undiscovered elements waiting to be added to the periodic table. Several factors can narrow down the field:
1. Spectroscopic Data: The Fingerprint of an Element
One of the most reliable methods for identifying an unknown element is through spectroscopic analysis. Different elements emit unique spectral signatures – characteristic patterns of light emitted when excited. If Part B provided spectral data (e.g., emission, absorption, or mass spectrometry data), analyzing these patterns could significantly aid in the identification of Element X.
- Emission Spectroscopy: This technique analyzes the light emitted by an excited element. Each element produces a unique pattern of bright lines, a kind of "fingerprint."
- Absorption Spectroscopy: This technique measures the light absorbed by an element at different wavelengths. This too creates a unique signature.
- Mass Spectrometry: This powerful technique measures the mass-to-charge ratio of ions, allowing for precise identification of isotopes and, consequently, the element itself.
Without the specific spectral data from Part B, however, we can only speculate on the potential matches.
2. Chemical Properties and Reactivity: Clues from Chemical Behavior
The chemical properties and reactivity of Element X provide invaluable clues. Did it react violently with water? Did it form stable oxides or halides? Did it exhibit catalytic properties? Each reaction sheds light on its position in the periodic table and its likely electronic configuration.
- Reactivity with Water: Highly reactive alkali metals and alkaline earth metals react explosively with water, while noble gases remain inert.
- Oxidation States: The number of oxidation states an element can exhibit reflects its electronic structure and its position in the periodic table.
- Formation of Compounds: The type of compounds formed (e.g., oxides, sulfides, halides) provides insights into its bonding characteristics and electronegativity.
Again, without specific details about the chemical behavior of Element X from Part B, it is impossible to confidently narrow down the possibilities.
3. Crystal Structure and Physical Properties: Solid-State Insights
The crystal structure of an element (how its atoms are arranged in a solid) offers crucial information about its bonding, density, and other physical properties. Techniques like X-ray diffraction can be used to determine the crystal structure. Physical properties such as melting point, boiling point, density, conductivity, and magnetism also help narrow down the possibilities.
- X-ray Diffraction: This technique uses X-rays to determine the arrangement of atoms in a crystalline solid.
- Density: Density provides information about the packing of atoms and the atomic weight of the element.
- Conductivity: Electrical and thermal conductivity can indicate whether the element is a metal, metalloid, or nonmetal.
Unfortunately, without data on the crystal structure and physical properties from Part B, this avenue of investigation remains speculative.
4. Isotopic Analysis: Unveiling Isotopic Signatures
Isotopes are atoms of the same element with different numbers of neutrons. The isotopic composition of an element can be determined using mass spectrometry. Certain elements have characteristic isotopic ratios that can help in their identification. Rare isotopes can also be used as tracers in various applications.
- Mass Spectrometry (again): Crucial for isotopic analysis.
- Isotope Ratio Mass Spectrometry (IRMS): A specialized technique for measuring very small differences in isotopic ratios.
Without Part B's isotopic analysis data, this line of investigation remains closed.
Challenges and Limitations in Identifying Element X
Several challenges hinder the unequivocal identification of Element X based solely on limited information. These include:
- Incomplete Data: The most significant challenge is the lack of complete data from Part B. Without comprehensive spectroscopic, chemical, and physical data, any conclusions remain tentative.
- Ambiguity in Descriptions: Vague or ambiguous descriptions of Element X's properties can lead to multiple interpretations and potential candidates.
- Interference from Other Elements: The presence of other elements in the sample could mask the true properties of Element X, leading to misidentification.
- Novel Elements: The possibility that Element X represents a completely new, undiscovered element adds complexity to the identification process. This would require synthesis and thorough characterization of the element itself.
Exploring Future Directions: A Path Forward
To definitively identify Element X, several steps are crucial:
- Access to Complete Data: Obtaining complete data from the original Part B experiment is paramount. This includes all spectroscopic data, chemical reactivity observations, physical properties measurements, and isotopic analysis results.
- Rigorous Data Analysis: Careful and rigorous analysis of the complete data set is essential to avoid misinterpretations.
- Cross-referencing with Existing Literature: Comparison of the collected data with existing literature on known and potentially undiscovered elements is crucial for narrowing down possibilities.
- Independent Verification: Independent researchers should replicate the experiments and analyze the data to verify the findings.
- Computational Modeling: Computational chemistry and materials science techniques can be used to predict the properties of potential candidates and compare them with the experimental data.
Conclusion: The Ongoing Mystery of Element X
The identity of Element X from Part B remains an intriguing scientific enigma. While we have explored several potential avenues of investigation, the lack of detailed information from Part B significantly hinders definitive identification. The future of unraveling this mystery lies in accessing complete data, applying rigorous analytical techniques, and exploring novel computational approaches. The pursuit of this knowledge continues to highlight the importance of detailed experimental design, precise data reporting, and collaborative efforts in scientific research. The quest for Element X's identity is a testament to the ongoing process of scientific discovery and the enduring fascination with the unknown. Until then, the mystery remains, tantalizing scientists and sparking further investigation into the fascinating world of elemental properties and chemical behavior.
Latest Posts
Latest Posts
-
Which Function Is Shown In The Graph Below
Mar 18, 2025
-
Endocytosis Moves Materials A Cell Via
Mar 18, 2025
-
Behaviorism Focuses On Making Psychology An Objective Science By
Mar 18, 2025
-
How To Link Chegg To Tinder
Mar 18, 2025
-
The Nucleus Of An Atom Contains
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Is The Identity Of Element X From Part B . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
