The Nucleus Of An Atom Contains
Holbox
Mar 18, 2025 · 7 min read
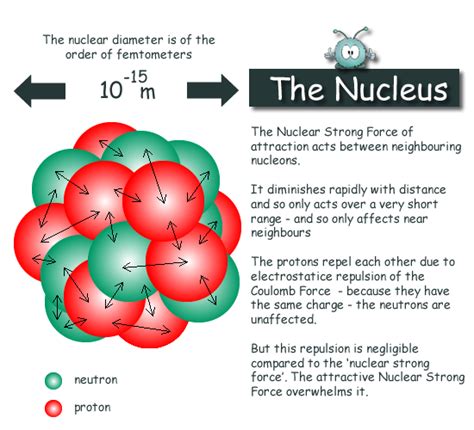
Table of Contents
The Nucleus of an Atom: A Deep Dive into its Composition and Significance
The atom, the fundamental building block of matter, is a fascinating world of its own. While often depicted as a simple sphere, the atom is a complex system composed of even smaller particles. At its heart lies the nucleus, a tiny, incredibly dense region that holds the key to an atom's identity and behavior. This article delves deep into the nucleus of an atom, exploring its composition, properties, and the crucial role it plays in various scientific fields.
What is the Atomic Nucleus?
The atomic nucleus is the positively charged center of an atom, containing most of its mass. It's a remarkably small region; if an atom were the size of a football stadium, the nucleus would be approximately the size of a pea in the center. This concentration of mass is what makes the nucleus so incredibly dense. Its density is on the order of 10<sup>17</sup> kg/m³, vastly exceeding the density of ordinary matter.
The Building Blocks of the Nucleus: Protons and Neutrons
The nucleus is composed of two types of subatomic particles:
Protons
Protons are positively charged particles with a mass approximately 1836 times greater than that of an electron. The number of protons in an atom's nucleus, known as its atomic number, uniquely identifies the element. For example, hydrogen has one proton (atomic number 1), helium has two (atomic number 2), and uranium has 92 (atomic number 92). This number dictates the element's chemical properties and its place on the periodic table.
Neutrons
Neutrons are electrically neutral particles with a mass slightly larger than that of a proton. Unlike protons, the number of neutrons in an atom's nucleus can vary, even within the same element. Atoms of the same element with different numbers of neutrons are called isotopes. For instance, carbon-12 has 6 protons and 6 neutrons, while carbon-14 has 6 protons and 8 neutrons. Isotopes exhibit similar chemical behavior but can differ significantly in their nuclear stability, impacting their radioactivity.
Nuclear Forces: Holding the Nucleus Together
The nucleus presents a fascinating paradox. Protons, being positively charged, should strongly repel each other due to the electromagnetic force. Yet, the nucleus remains stable. This stability is attributed to the strong nuclear force, a fundamental force of nature that is much stronger than the electromagnetic force at short distances. This force overcomes the electrostatic repulsion between protons and binds the protons and neutrons together within the nucleus.
The strong nuclear force is short-ranged; it acts only over distances comparable to the size of the nucleus. Beyond this range, the electromagnetic force dominates. The balance between the strong nuclear force and the electromagnetic force is crucial for the stability of the nucleus. If the electromagnetic repulsion becomes too strong relative to the nuclear force, the nucleus can become unstable and undergo radioactive decay.
Nuclear Stability and Radioactive Decay
Nuclear stability is a critical concept in understanding atomic nuclei. A nucleus is considered stable if it does not spontaneously transform into another nucleus. Many isotopes are stable, while others are unstable and undergo radioactive decay.
Radioactive decay involves the emission of particles or energy from an unstable nucleus to achieve a more stable configuration. There are several types of radioactive decay:
- Alpha decay: Emission of an alpha particle (two protons and two neutrons).
- Beta decay: Emission of a beta particle (an electron or a positron).
- Gamma decay: Emission of a gamma ray (high-energy photon).
The rate of radioactive decay is characterized by the half-life, which is the time it takes for half of a given amount of a radioactive isotope to decay. Half-lives can range from fractions of a second to billions of years. Radioactive decay is used in various applications, including medical imaging, carbon dating, and nuclear power generation.
Nuclear Fission and Fusion
The immense energy stored within atomic nuclei can be released through two primary processes: nuclear fission and nuclear fusion.
Nuclear Fission
Nuclear fission is the splitting of a heavy nucleus into two lighter nuclei. This process releases a tremendous amount of energy, as some of the mass is converted into energy according to Einstein's famous equation, E=mc². Nuclear fission is the basis for nuclear power plants and nuclear weapons.
Nuclear Fusion
Nuclear fusion is the process of combining two light nuclei to form a heavier nucleus. This process also releases a large amount of energy, even greater than fission. The sun's energy is produced through nuclear fusion reactions, where hydrogen nuclei fuse to form helium. Controlled nuclear fusion is a major area of research, aiming to create a clean and virtually limitless energy source.
The Nucleus and the Periodic Table
The atomic nucleus plays a central role in the organization of the periodic table of elements. The periodic table arranges elements based on their atomic number, which is determined by the number of protons in the nucleus. Elements with similar chemical properties are grouped together in columns, reflecting their electron configurations, which are in turn determined by the nuclear charge.
The arrangement of elements in the periodic table reflects recurring patterns in their electron configurations and, consequently, their chemical behavior. This pattern emerges from the underlying structure of the atom's nucleus and the interactions of its protons and neutrons.
The Significance of Nuclear Studies
The study of atomic nuclei has profound implications across many scientific disciplines. Some key areas include:
- Nuclear Physics: This field focuses on understanding the structure and properties of atomic nuclei, including their stability, decay processes, and interactions with other particles.
- Nuclear Chemistry: This field explores the chemical behavior of radioactive isotopes and their applications in various areas, such as medical imaging, environmental monitoring, and industrial processes.
- Nuclear Medicine: This field utilizes radioactive isotopes for diagnostic and therapeutic purposes, including cancer treatment and imaging techniques such as PET scans.
- Nuclear Engineering: This field focuses on the design and operation of nuclear reactors, as well as the development of new applications for nuclear energy.
- Cosmology and Astrophysics: Nuclear processes play a vital role in the formation and evolution of stars and galaxies. Understanding nuclear reactions is crucial for modeling stellar evolution and understanding the origin of elements in the universe.
- Geochronology: Radioactive decay is a crucial tool for determining the age of rocks, fossils, and other materials, providing insights into the Earth's history and the evolution of life.
Nuclear Structure and Models
Several models have been developed to describe the structure of the atomic nucleus. These models attempt to explain the observed properties of nuclei, such as their size, mass, stability, and energy levels. Some of the prominent models include:
- The Liquid Drop Model: This model views the nucleus as a drop of incompressible liquid, with protons and neutrons interacting through short-range forces. This model successfully explains the mass defects and binding energies of many nuclei.
- The Shell Model: This model considers the protons and neutrons to occupy discrete energy levels or shells, similar to electrons in an atom. This model successfully explains the magic numbers of nucleons (protons and neutrons) that correspond to particularly stable nuclei.
- The Collective Model: This model combines features of the liquid drop model and the shell model, accounting for both collective vibrations and individual particle motions within the nucleus.
Future Directions in Nuclear Research
Nuclear research continues to be an active and important area of scientific inquiry. Ongoing research focuses on several key areas:
- Understanding the fundamental forces: Deeper understanding of the strong and weak nuclear forces is crucial for refining nuclear models and predicting nuclear properties.
- Exploring new isotopes: Synthesizing and characterizing new, exotic isotopes expands our knowledge of nuclear structure and stability.
- Developing new nuclear technologies: This includes advancing nuclear medicine techniques, improving nuclear energy efficiency and safety, and exploring potential applications in other areas.
- Nuclear waste management: Developing safe and efficient methods for handling and disposing of nuclear waste is a critical challenge.
In conclusion, the nucleus of an atom, a tiny but powerful entity, is a captivating subject of study. Its composition, properties, and the forces governing its behavior are fundamental to our understanding of matter and energy. From the organization of the periodic table to the power generation of stars and the development of life-saving medical technologies, the nucleus plays a crucial role in shaping our world. Ongoing research continues to unravel its mysteries, offering exciting possibilities for the future.
Latest Posts
Latest Posts
-
A Section Of Dna Has The Base Sequence Shown In
Mar 18, 2025
-
Draw The Shear And Moment Diagrams For The Beam Chegg
Mar 18, 2025
-
Draw The Shear Diagram For The Beam Chegg
Mar 18, 2025
-
Eating Soup That Has Been Time Temperature Abused Can Result In
Mar 18, 2025
-
Can You Write In Books Rented From Chegg
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about The Nucleus Of An Atom Contains . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
