What Is The Correct Classification Of The Following Reaction
Holbox
Apr 02, 2025 · 6 min read
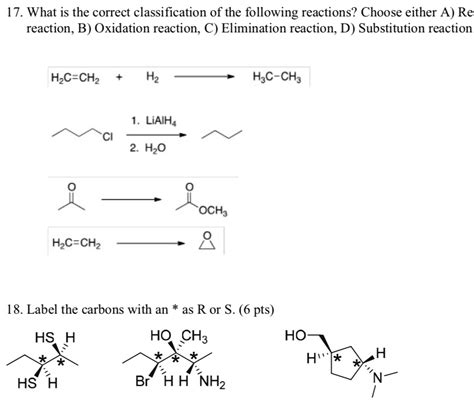
Table of Contents
- What Is The Correct Classification Of The Following Reaction
- Table of Contents
- What is the correct classification of the following reaction? A Comprehensive Guide to Reaction Classification
- The Five Main Types of Chemical Reactions
- Beyond the Basic Five: More Complex Classifications
- Identifying Reaction Types: A Step-by-Step Approach
- Conclusion: Mastering Reaction Classification
- Latest Posts
- Latest Posts
- Related Post
What is the correct classification of the following reaction? A Comprehensive Guide to Reaction Classification
Classifying chemical reactions is crucial for understanding their mechanisms, predicting their products, and applying them in various fields like synthesis, analysis, and industrial processes. There's no single "following reaction" provided, so this article will explore the major categories of chemical reactions and the criteria used for classification, equipping you with the tools to classify any given reaction. We'll delve into examples and nuances to solidify your understanding.
The Five Main Types of Chemical Reactions
Chemical reactions are broadly categorized into five main types:
-
Combination Reactions (Synthesis Reactions): In these reactions, two or more reactants combine to form a single product. The general form is A + B → AB.
- Example: The formation of water from hydrogen and oxygen: 2H₂ + O₂ → 2H₂O. This is a classic example of a combination reaction where two elements combine to form a compound.
- Example: The reaction of calcium oxide with carbon dioxide to produce calcium carbonate: CaO + CO₂ → CaCO₃. This exemplifies the combination of two compounds to form a single, more complex compound.
-
Decomposition Reactions: These are the reverse of combination reactions. A single reactant breaks down into two or more simpler products. The general form is AB → A + B.
- Example: The decomposition of calcium carbonate upon heating: CaCO₃ → CaO + CO₂. Heat provides the energy needed to break the bonds in calcium carbonate, leading to the formation of calcium oxide and carbon dioxide.
- Example: The electrolysis of water: 2H₂O → 2H₂ + O₂. An electric current provides the energy to decompose water into its constituent elements, hydrogen and oxygen. This reaction is also significant in demonstrating the production of hydrogen as a potential fuel source.
-
Single Displacement Reactions (Substitution Reactions): In these reactions, a more reactive element replaces a less reactive element in a compound. The general form is A + BC → AC + B.
- Example: The reaction of zinc with hydrochloric acid: Zn + 2HCl → ZnCl₂ + H₂. Zinc, being more reactive than hydrogen, displaces hydrogen from hydrochloric acid, forming zinc chloride and hydrogen gas. This reaction is frequently used to generate hydrogen gas in the laboratory.
- Example: The reaction of iron with copper(II) sulfate: Fe + CuSO₄ → FeSO₄ + Cu. Iron, being more reactive than copper, displaces copper from copper(II) sulfate, forming iron(II) sulfate and copper metal. This reaction showcases the relative reactivities of metals. The reactivity series plays a crucial role in predicting the outcome of such reactions.
-
Double Displacement Reactions (Metathesis Reactions): These reactions involve the exchange of ions between two compounds, typically in aqueous solution. The general form is AB + CD → AD + CB.
- Example: The reaction of silver nitrate with sodium chloride: AgNO₃ + NaCl → AgCl + NaNO₃. Silver chloride, an insoluble precipitate, forms, driving the reaction forward. This reaction is a classic example of a precipitation reaction, a subset of double displacement reactions.
- Example: The reaction of hydrochloric acid with sodium hydroxide: HCl + NaOH → NaCl + H₂O. This is an acid-base neutralization reaction, another important type of double displacement reaction, where an acid and a base react to form salt and water. Understanding acid-base chemistry is essential for classifying and predicting the outcomes of these reactions. The concept of pH and its measurement are critical to comprehending neutralization reactions.
-
Combustion Reactions: These reactions involve the rapid reaction of a substance with oxygen, usually producing heat and light. They are often exothermic, releasing significant energy.
- Example: The combustion of methane: CH₄ + 2O₂ → CO₂ + 2H₂O. This reaction is commonly used for generating heat and electricity, showcasing its practical significance. Understanding stoichiometry is key in predicting the amounts of reactants and products involved in combustion.
- Example: The combustion of propane: C₃H₈ + 5O₂ → 3CO₂ + 4H₂O. Propane is another common fuel, and its combustion follows the same principles as methane combustion. The balancing of chemical equations is essential for correctly representing combustion reactions and other chemical processes.
Beyond the Basic Five: More Complex Classifications
While these five categories cover most common reactions, some reactions defy simple classification or exhibit characteristics of multiple types. Several other classifications exist, adding further layers of understanding:
-
Redox Reactions (Oxidation-Reduction Reactions): These reactions involve the transfer of electrons between species. One species undergoes oxidation (loss of electrons), while another undergoes reduction (gain of electrons). Many reactions, including combustion and single displacement reactions, are also redox reactions.
- Example: The rusting of iron: 4Fe + 3O₂ → 2Fe₂O₃. Iron is oxidized (loses electrons), and oxygen is reduced (gains electrons). Understanding oxidation states is critical for classifying redox reactions.
- Example: The reaction between zinc and copper(II) ions: Zn + Cu²⁺ → Zn²⁺ + Cu. Zinc is oxidized, and copper(II) ions are reduced. This is a classic example of a redox reaction used in electrochemical cells (batteries).
-
Acid-Base Reactions: These reactions involve the transfer of protons (H⁺ ions) between an acid (proton donor) and a base (proton acceptor). Neutralization reactions are a specific type of acid-base reaction.
- Example: The reaction of acetic acid with sodium hydroxide: CH₃COOH + NaOH → CH₃COONa + H₂O. This shows the neutralization of a weak acid (acetic acid) by a strong base (sodium hydroxide).
-
Precipitation Reactions: A subset of double displacement reactions, these reactions form a solid precipitate (insoluble compound) when two aqueous solutions are mixed.
- Example: (Already mentioned above) AgNO₃ + NaCl → AgCl + NaNO₃. The formation of the insoluble silver chloride precipitate is the driving force behind this reaction.
-
Neutralization Reactions: (Already discussed above) These are acid-base reactions that result in the formation of salt and water.
Identifying Reaction Types: A Step-by-Step Approach
To correctly classify a reaction, follow these steps:
-
Identify the reactants and products: Write down the chemical formulas of all the reactants and products involved in the reaction.
-
Observe changes in the number of reactants and products: Does the reaction involve the combination of multiple reactants to form a single product (combination)? Does a single reactant break down into multiple products (decomposition)? Does one element replace another in a compound (single displacement)? Are ions exchanged between two compounds (double displacement)? Does a substance react rapidly with oxygen, producing heat and light (combustion)?
-
Analyze electron transfer: Does the reaction involve the transfer of electrons (redox)? Look for changes in oxidation states.
-
Analyze proton transfer: Does the reaction involve the transfer of protons (acid-base)? Look for the presence of acids and bases.
-
Consider solubility: Does the reaction produce a solid precipitate (precipitation)?
-
Combine observations: Based on your observations, classify the reaction according to the most appropriate category. Some reactions may fit multiple categories (e.g., a redox reaction that is also a single displacement reaction).
Conclusion: Mastering Reaction Classification
Classifying chemical reactions is a fundamental skill in chemistry. Understanding the different types of reactions, their mechanisms, and the criteria for classification allows for accurate predictions of reaction products, the design of efficient synthetic pathways, and the interpretation of experimental observations. By applying the steps outlined above and familiarizing yourself with the numerous examples provided, you'll develop a strong foundation for classifying any chemical reaction you encounter. Remember to always consider the specific details of the reaction, as some reactions may display characteristics of multiple classifications. Consistent practice and careful observation are key to mastering this essential aspect of chemistry.
Latest Posts
Latest Posts
-
What Is The Formula For The Compound Iron Iii Sulfite
Apr 05, 2025
-
Tom Has Built A Large Slingshot
Apr 05, 2025
-
As A Hiker In Glacier National Park
Apr 05, 2025
-
3 Rs For Responding To Aggressive Behavior
Apr 05, 2025
-
A Mathematical Sentence With An Equal Symbol Used
Apr 05, 2025
Related Post
Thank you for visiting our website which covers about What Is The Correct Classification Of The Following Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
