What Is The Formula For The Compound Iron Iii Sulfite
Holbox
Apr 05, 2025 · 6 min read
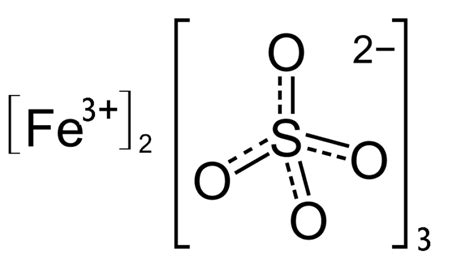
Table of Contents
- What Is The Formula For The Compound Iron Iii Sulfite
- Table of Contents
- What is the Formula for the Compound Iron(III) Sulfite?
- Understanding the Components: Iron(III) and Sulfite
- Iron(III) (Fe³⁺)
- Sulfite (SO₃²⁻)
- Determining the Formula: Charge Balance is Key
- Considering Hydration: The Role of Water Molecules
- Synthesis and Stability Challenges: Why it's Difficult to Obtain Pure Iron(III) Sulfite
- Characterization Techniques: Identifying and Quantifying Iron(III) Sulfite
- Applications and Significance: Why Study Iron(III) Sulfite?
- Conclusion: A Complex Compound with Significant Implications
- Latest Posts
- Latest Posts
- Related Post
What is the Formula for the Compound Iron(III) Sulfite?
Iron(III) sulfite, a fascinating inorganic compound, presents a unique challenge in determining its precise chemical formula. Unlike many simpler ionic compounds, its formula isn't immediately obvious due to the potential for varying degrees of hydration and the complex nature of sulfite ion's behavior. This article will delve into the intricacies of determining the formula, exploring the underlying chemical principles involved and considering the various forms this compound can take.
Understanding the Components: Iron(III) and Sulfite
Before tackling the formula, let's examine the individual components:
Iron(III) (Fe³⁺)
Iron(III), also known as ferric iron, carries a +3 charge. This positive charge stems from the loss of three electrons from a neutral iron atom. This cation is crucial in determining the overall charge balance in the compound.
Sulfite (SO₃²⁻)
The sulfite ion (SO₃²⁻) is a polyatomic anion with a -2 charge. It consists of a central sulfur atom bonded to three oxygen atoms. The negative charge arises from the formal oxidation state of sulfur being +4 (rather than the +6 in sulfate, SO₄²⁻). The sulfite ion's reactivity and its tendency to undergo oxidation (losing electrons and becoming sulfate) are significant factors to consider when dealing with iron(III) sulfite.
Determining the Formula: Charge Balance is Key
The fundamental principle in determining the formula of any ionic compound is the law of conservation of charge. The overall charge of the compound must be neutral. This means the positive charges from the cations must exactly balance the negative charges from the anions.
To achieve neutrality in iron(III) sulfite, we need to find the ratio of Fe³⁺ and SO₃²⁻ ions that cancels out the charges. We can use a simple algebraic approach:
Let's assume 'x' represents the number of Fe³⁺ ions and 'y' represents the number of SO₃²⁻ ions. The charge balance equation would be:
3x + (-2y) = 0
To solve for the simplest whole-number ratio, we can rearrange the equation:
3x = 2y
x/y = 2/3
This tells us that for every two Fe³⁺ ions, we need three SO₃²⁻ ions to achieve charge neutrality. Therefore, the basic formula for anhydrous iron(III) sulfite is Fe₂(SO₃)₃.
Considering Hydration: The Role of Water Molecules
The formula Fe₂(SO₃)₃ represents the anhydrous form – meaning it contains no water molecules. However, many inorganic salts readily incorporate water molecules into their crystal structure, forming hydrates. Iron(III) sulfite is no exception. It can exist as various hydrates, with the general formula Fe₂(SO₃)₃·xH₂O, where 'x' represents the number of water molecules associated with each formula unit. The value of 'x' can vary depending on the conditions under which the compound is prepared.
The presence of water molecules significantly affects the compound's properties, including its solubility, color, and stability. Determining the exact value of 'x' often requires experimental techniques like thermogravimetric analysis (TGA), which measures weight loss as a function of temperature to determine the amount of water driven off.
Synthesis and Stability Challenges: Why it's Difficult to Obtain Pure Iron(III) Sulfite
Synthesizing pure, well-defined iron(III) sulfite presents considerable challenges. This difficulty is primarily due to the following factors:
-
Sulfite's Instability: The sulfite ion (SO₃²⁻) is relatively unstable and readily undergoes oxidation to sulfate (SO₄²⁻) in the presence of oxygen. This oxidation is even more pronounced in the presence of a relatively strong oxidizing agent such as Fe³⁺. Therefore, carefully controlled, anaerobic conditions are crucial during synthesis to minimize sulfate formation.
-
Hydrolysis: Iron(III) ions are prone to hydrolysis in aqueous solutions, reacting with water to form various iron(III) hydroxide species. This hydrolysis competes with the formation of the desired sulfite compound, leading to impure products.
-
Hydration Variability: As mentioned earlier, the formation of hydrates with varying amounts of water makes it difficult to obtain a consistently anhydrous form. The degree of hydration depends on the conditions such as temperature, humidity, and the concentration of reactants.
These challenges highlight why obtaining a pure sample of iron(III) sulfite with a precisely defined hydration state is a significant undertaking in experimental inorganic chemistry.
Characterization Techniques: Identifying and Quantifying Iron(III) Sulfite
Various analytical techniques are employed to characterize iron(III) sulfite and its hydrates. These techniques help establish the chemical composition and confirm the presence or absence of impurities:
-
X-ray Diffraction (XRD): This technique provides information about the crystal structure of the compound, including the arrangement of atoms and the presence of hydrated water molecules. It can distinguish between different hydrate forms and identify any crystalline impurities.
-
Thermogravimetric Analysis (TGA): As mentioned above, TGA can precisely determine the amount of water present in a hydrated sample by measuring the weight loss upon heating. This data helps determine the value of 'x' in the formula Fe₂(SO₃)₃·xH₂O.
-
Infrared Spectroscopy (IR): IR spectroscopy identifies the presence of specific functional groups and bonds within the molecule. It can confirm the presence of sulfite ions (SO₃²⁻) and distinguish it from sulfate (SO₄²⁻).
-
Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES): This powerful technique measures the concentration of elemental components, such as iron and sulfur, in the sample. This helps to verify the stoichiometry of the compound and detect any impurities.
Applications and Significance: Why Study Iron(III) Sulfite?
Despite its synthesis challenges, iron(III) sulfite and related compounds hold potential applications and are important for understanding several chemical and environmental processes:
-
Water Treatment: Iron(III) compounds are utilized as coagulants and flocculants in water treatment to remove impurities. While sulfite itself is not typically used directly in this context, its reactions and behavior are relevant in understanding the chemistry of these processes.
-
Catalysis: Iron compounds are used in various catalytic processes, and understanding the reactivity of iron(III) sulfite could provide insights into the design of new and improved catalysts.
-
Environmental Chemistry: The oxidation of sulfite to sulfate is a significant process in atmospheric and aquatic environments. The behavior of iron(III) in these processes is crucial in understanding the fate of sulfur-containing pollutants.
-
Materials Science: Iron sulfite could potentially be used as a precursor in the synthesis of other iron-sulfur containing materials with specific applications.
Conclusion: A Complex Compound with Significant Implications
The formula for iron(III) sulfite, Fe₂(SO₃)₃, represents the anhydrous form of the compound. However, the likelihood of obtaining a pure, anhydrous sample is low due to the instability of the sulfite ion and the tendency for hydration and hydrolysis to occur. Understanding the synthesis challenges and utilizing characterization techniques is crucial in studying this compound and its various hydrated forms. Although challenging to synthesize in a pure form, iron(III) sulfite holds implications in several fields and contributes to a broader understanding of iron chemistry and sulfur cycling in various environmental and industrial processes. Further research and advanced synthetic methodologies are required to fully explore the potential applications and properties of this fascinating inorganic compound.
Latest Posts
Latest Posts
-
Tirri Corporation Has Provided The Following Information
Apr 11, 2025
-
The Depreciation Tax Shield Is Best Defined As The
Apr 11, 2025
-
Excel 2021 Skills Approach Ch 3 Fix It 3 6
Apr 11, 2025
-
What Is True About The Electron Transport Chain
Apr 11, 2025
-
A Functional Structure Is Recommended When A Firm
Apr 11, 2025
Related Post
Thank you for visiting our website which covers about What Is The Formula For The Compound Iron Iii Sulfite . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.