What Is Meant By The Simplest Formula Of A Compound
Holbox
Mar 15, 2025 · 5 min read
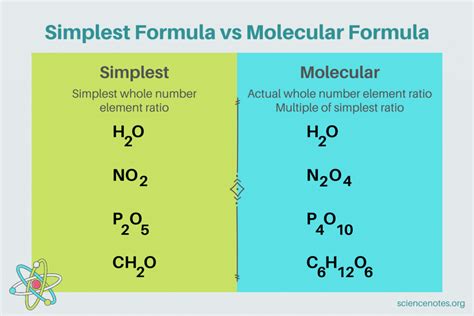
Table of Contents
What is Meant by the Simplest Formula of a Compound?
Understanding the simplest formula of a compound is fundamental to chemistry. It's a cornerstone concept that underpins our ability to describe, analyze, and predict the behavior of matter. This article will delve deep into the meaning of the simplest formula, also known as the empirical formula, exploring its derivation, applications, and crucial distinctions from other chemical formulas.
Defining the Simplest Formula (Empirical Formula)
The simplest formula of a compound, more formally known as the empirical formula, represents the simplest whole-number ratio of atoms of each element present in a compound. It's the most reduced form of a chemical formula, showing the smallest possible integer values for the subscripts representing the number of atoms of each element. Unlike the molecular formula, which shows the exact number of atoms of each element in a molecule, the empirical formula only indicates the ratio of these atoms.
For example, consider glucose, a simple sugar. Its molecular formula is C₆H₁₂O₆, indicating six carbon atoms, twelve hydrogen atoms, and six oxygen atoms per molecule. However, the empirical formula for glucose is CH₂O. This is because the ratio of carbon to hydrogen to oxygen atoms is 1:2:1, which is the simplest whole-number ratio that can be expressed.
Key difference: The empirical formula represents the ratio, while the molecular formula represents the actual number of atoms in a molecule. Many compounds share the same empirical formula but have different molecular formulas.
Determining the Simplest Formula: A Step-by-Step Guide
Determining the simplest formula often involves experimental data, typically from elemental analysis or combustion analysis. Here's a detailed walkthrough:
-
Determine the mass of each element in the compound: This is usually obtained through experimental techniques like gravimetric analysis or instrumental methods such as atomic absorption spectroscopy (AAS) or inductively coupled plasma mass spectrometry (ICP-MS). The results are usually expressed as percentages by mass.
-
Convert the mass of each element to moles: Use the molar mass (atomic weight) of each element to convert the mass (in grams) to moles using the following formula:
Moles = Mass (g) / Molar Mass (g/mol)
-
Determine the mole ratio of each element: Divide the number of moles of each element by the smallest number of moles obtained in step 2. This will give you the simplest whole-number ratio of atoms.
-
Express the simplest formula: Use the whole-number mole ratios obtained in step 3 as subscripts for the chemical symbols of each element to write the empirical formula. If the ratios are not whole numbers, you may need to multiply all ratios by a small integer to obtain whole numbers. For instance, if you get a ratio of 1.5:1, multiply both by 2 to get 3:2.
Illustrative Example:
Let's say we have a compound containing 40.0% carbon, 6.7% hydrogen, and 53.3% oxygen by mass. Let's find its empirical formula.
-
Mass to Moles:
- Carbon: (40.0 g / 12.01 g/mol) = 3.33 mol
- Hydrogen: (6.7 g / 1.01 g/mol) = 6.63 mol
- Oxygen: (53.3 g / 16.00 g/mol) = 3.33 mol
-
Mole Ratio: Divide each mole value by the smallest number of moles (3.33 mol):
- Carbon: 3.33 mol / 3.33 mol = 1.00
- Hydrogen: 6.63 mol / 3.33 mol ≈ 2.00
- Oxygen: 3.33 mol / 3.33 mol = 1.00
-
Empirical Formula: The simplest whole-number ratio is 1:2:1. Therefore, the empirical formula is CH₂O.
Distinguishing Between Empirical and Molecular Formulas
The distinction between the empirical formula and the molecular formula is crucial. The empirical formula shows the simplest ratio, while the molecular formula shows the actual number of atoms in a molecule.
-
Empirical Formula: Represents the simplest whole-number ratio of atoms in a compound.
-
Molecular Formula: Represents the actual number of atoms of each element in a molecule of the compound. It is a multiple of the empirical formula.
To determine the molecular formula, you need additional information, such as the molar mass of the compound. You can then calculate the ratio between the molar mass of the empirical formula and the molar mass of the compound. This ratio is then used to multiply the subscripts in the empirical formula to obtain the molecular formula.
For example, if the empirical formula is CH₂O (like in the glucose example above), and the molar mass of the compound is determined to be 180 g/mol, the molar mass of CH₂O is 30 g/mol (12.01 + 2(1.01) + 16.00). The ratio is 180 g/mol / 30 g/mol = 6. Therefore, the molecular formula is (CH₂O)₆ = C₆H₁₂O₆.
Applications of the Simplest Formula
The simplest formula has widespread applications across various chemical fields:
-
Qualitative Analysis: Identifying the elements present in a compound.
-
Quantitative Analysis: Determining the relative amounts of each element in a compound.
-
Chemical Reactions: Balancing chemical equations and predicting stoichiometry.
-
Organic Chemistry: Determining the composition of unknown organic compounds.
-
Polymer Chemistry: Characterizing the repeating units in polymers.
Advanced Considerations: Dealing with Non-Whole Number Ratios
Sometimes, the mole ratios calculated from experimental data aren't perfect whole numbers. This could be due to experimental error or the inherent nature of the compound. In such cases, you might need to round the ratios to the nearest whole number. However, if the deviation from a whole number is significant, you might need to multiply all the ratios by a small integer to obtain whole numbers. This requires careful judgment based on the experimental accuracy and the context of the problem.
Conclusion: The Foundation of Chemical Understanding
The simplest formula, or empirical formula, represents a fundamental concept in chemistry. Its determination relies on meticulous experimental procedures and careful calculations. Understanding its significance and the crucial distinction between it and the molecular formula is essential for grasping the composition and behavior of chemical compounds. This seemingly simple concept serves as a building block for more advanced chemical studies and is a cornerstone of chemical analysis and understanding. Mastering the techniques associated with determining the simplest formula equips one with the skills to navigate the complexities of the chemical world.
Latest Posts
Latest Posts
-
For The Hr Planning Process How Should Goals Be Determined
Mar 17, 2025
-
How Does A Shortcut Link To Another File
Mar 17, 2025
-
Cash Flows From Financing Activities Do Not Include
Mar 17, 2025
-
A Positive Return On Investment For Education Happens When
Mar 17, 2025
-
What Is The Value Of I
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is Meant By The Simplest Formula Of A Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
