What Is An Accurate Description Of The Silicon Oxygen Tetrahedron
Holbox
Mar 22, 2025 · 5 min read
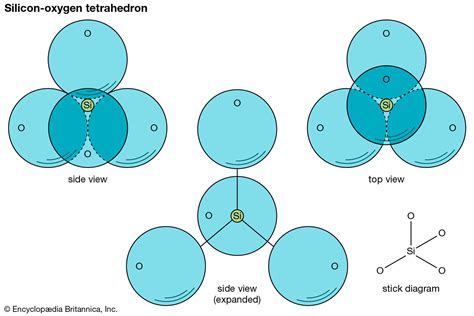
Table of Contents
- What Is An Accurate Description Of The Silicon Oxygen Tetrahedron
- Table of Contents
- What is an Accurate Description of the Silicon-Oxygen Tetrahedron?
- The Structure: A Foundation of Silicate Minerals
- Silicon's Bonding Behavior
- Oxygen's Role in the Structure
- Bonding Characteristics: More Than Just Covalent Bonds
- Polarity and its Consequences
- Variations and Polymorphism: Building with Tetrahedra
- Isolated Tetrahedra: Simple Beginnings
- Single Chain Silicates: Linear Arrangements
- Double Chain Silicates: More Complex Linkages
- Sheet Silicates: Two-Dimensional Networks
- Framework Silicates: Three-Dimensional Networks
- Importance in Different Contexts: A Multifaceted Role
- Geology and Petrology: Understanding Earth's Composition
- Materials Science: Designing Advanced Materials
- Environmental Science: Weathering and Soil Formation
- Biology: Unexpected Roles
- Conclusion: A Ubiquitous and Essential Structure
- Latest Posts
- Latest Posts
- Related Post
What is an Accurate Description of the Silicon-Oxygen Tetrahedron?
The silicon-oxygen tetrahedron is a fundamental building block in numerous minerals and materials, playing a crucial role in geology, materials science, and even biology. Understanding its structure and properties is key to comprehending the behavior of a vast array of substances. This article provides a comprehensive overview of the silicon-oxygen tetrahedron, delving into its structure, bonding characteristics, variations, and importance in different contexts.
The Structure: A Foundation of Silicate Minerals
At its core, the silicon-oxygen tetrahedron is a geometric shape composed of one silicon (Si) atom surrounded by four oxygen (O) atoms. The silicon atom sits at the center, while the four oxygen atoms occupy the four corners of a tetrahedron – a three-dimensional shape with four triangular faces. This arrangement is not arbitrary; it's a direct consequence of the electronic configuration and bonding preferences of silicon and oxygen.
Silicon's Bonding Behavior
Silicon, a group 14 element, possesses four valence electrons. To achieve a stable octet, it readily forms four covalent bonds. Each bond involves the sharing of an electron pair between silicon and oxygen. This covalent bonding is strong and crucial to the tetrahedron's stability.
Oxygen's Role in the Structure
Oxygen, a group 16 element, has six valence electrons and needs two more to complete its octet. Therefore, each oxygen atom in the tetrahedron forms a covalent bond with the central silicon atom and can form another bond with another cation, an anion, or even another silicon-oxygen tetrahedron. This ability to link tetrahedra is fundamental to the diversity of silicate structures.
Bonding Characteristics: More Than Just Covalent Bonds
While the Si-O bonds are primarily covalent, the character of the bonds is not purely covalent. There's a significant degree of ionic character involved due to the difference in electronegativity between silicon (1.8) and oxygen (3.5). Oxygen, being more electronegative, attracts the shared electron pair more strongly, resulting in a partial negative charge (δ-) on oxygen and a partial positive charge (δ+) on silicon. This ionic contribution influences the overall properties of the tetrahedron and the materials it forms.
Polarity and its Consequences
The polar nature of the Si-O bonds leads to several important consequences. The tetrahedron possesses a dipole moment, meaning it has a positive and a negative end. This polarity influences how tetrahedra interact with each other and other ions within a mineral structure. It also affects the physical and chemical properties of silicate minerals, such as their melting points, solubility, and reactivity.
Variations and Polymorphism: Building with Tetrahedra
The versatility of the silicon-oxygen tetrahedron lies in its ability to link with itself in various ways, creating a vast array of silicate structures. These structures are classified based on how the tetrahedra are linked:
Isolated Tetrahedra: Simple Beginnings
In some silicate minerals, the tetrahedra exist as independent units, not bonded to each other. These are known as nesosilicates. Olivine, a common mineral in the Earth's mantle, is an example of a mineral with isolated tetrahedra. The tetrahedra are held together by other cations in the structure, such as magnesium (Mg) and iron (Fe).
Single Chain Silicates: Linear Arrangements
In inosilicates, tetrahedra share two oxygen atoms, forming continuous chains. Pyroxenes, a group of important rock-forming minerals, are prime examples of this structure. The chains can be single or double, leading to variations in the overall mineral structure.
Double Chain Silicates: More Complex Linkages
Amphiboles, another significant group of rock-forming minerals, exhibit double chains of tetrahedra. These double chains are formed by sharing two and three oxygen atoms between adjacent tetrahedra, resulting in a more complex and robust structure compared to single chains.
Sheet Silicates: Two-Dimensional Networks
Phyllosilicates, also known as sheet silicates, form two-dimensional sheets of linked tetrahedra. Each tetrahedron shares three of its oxygen atoms with neighboring tetrahedra, creating a continuous plane. This arrangement leads to minerals like mica and clay minerals, characterized by their characteristic cleavage along these sheets. The weak bonding between the sheets accounts for their easy splitting.
Framework Silicates: Three-Dimensional Networks
Tectosilicates represent the most complex arrangement, where each tetrahedron shares all four of its oxygen atoms with neighboring tetrahedra. This creates a three-dimensional framework structure. Quartz (SiO2) and feldspars are classic examples of tectosilicates. The strong, interconnected network results in hard, durable minerals.
Importance in Different Contexts: A Multifaceted Role
The silicon-oxygen tetrahedron's impact extends far beyond mineralogy. Its significance can be seen in various scientific fields:
Geology and Petrology: Understanding Earth's Composition
Silicate minerals, formed from linked silicon-oxygen tetrahedra, constitute the vast majority of the Earth's crust and mantle. Understanding their structure and behavior is crucial for comprehending plate tectonics, volcanic activity, and the formation of various rocks.
Materials Science: Designing Advanced Materials
The properties of silicate materials can be tailored by manipulating the arrangement of silicon-oxygen tetrahedra. This allows materials scientists to design advanced materials with specific properties for applications in construction, electronics, and many other fields. Ceramics, glasses, and zeolites are examples of materials utilizing this principle.
Environmental Science: Weathering and Soil Formation
The weathering and breakdown of silicate minerals significantly impact soil formation and nutrient cycling in ecosystems. The structure of the silicon-oxygen tetrahedron influences the rate at which minerals weather, releasing essential nutrients for plant growth.
Biology: Unexpected Roles
While not as prominent as in geology and materials science, silicon-oxygen tetrahedra play a role in some biological systems. Diatoms, single-celled algae, use silica to construct their intricate cell walls, which are made up of amorphous silica, a non-crystalline form of silicon dioxide.
Conclusion: A Ubiquitous and Essential Structure
The silicon-oxygen tetrahedron, seemingly simple in its structure, is a cornerstone of our physical world. Its ability to link in diverse ways gives rise to an immense variety of minerals and materials, with properties ranging from the brittle hardness of quartz to the flexible layers of mica. From the Earth's deepest depths to the smallest biological organisms, this fundamental structure continues to shape our environment and inspire innovative applications in various scientific and technological domains. Further research into its behavior and potential remains a crucial area of scientific exploration.
Latest Posts
Latest Posts
-
What Is The Appropriate Description For A Plant Processing Franchise
Mar 23, 2025
-
Advertising Can Impede Economic Efficiency When It
Mar 23, 2025
-
How Is A Cost Leader Protected From Threats From Powerful Suppliers
Mar 23, 2025
-
Label The Photomicrograph Of Thick Skin
Mar 23, 2025
-
Human Pathogens Fall Into The Group
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about What Is An Accurate Description Of The Silicon Oxygen Tetrahedron . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
