Water Is Always A Product In What Type Of Reaction
Holbox
Mar 12, 2025 · 5 min read
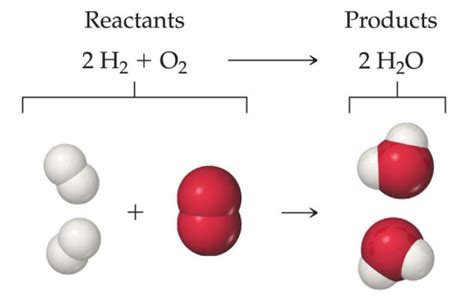
Table of Contents
Water is Always a Product in What Type of Reaction? Understanding Synthesis and Decomposition Reactions
Water (H₂O), a ubiquitous and essential molecule, plays a crucial role in numerous chemical reactions. While it can act as a reactant in some processes, it's particularly prominent as a product in specific types of reactions. This article delves deep into the world of chemical reactions, focusing on those where water is invariably formed: synthesis reactions and specifically, acid-base neutralization reactions, a subset of synthesis reactions, and the reverse – decomposition reactions where water is a reactant that breaks down to form other products.
Understanding Synthesis Reactions
Synthesis reactions, also known as combination reactions, are fundamentally characterized by the formation of a more complex compound from two or more simpler substances. These simpler substances can be elements or simpler compounds. The general form of a synthesis reaction can be represented as:
A + B → AB
where A and B are reactants, and AB is the product formed by their combination. Numerous synthesis reactions result in water as a byproduct. Let's explore some key examples:
Acid-Base Neutralization Reactions: The Most Common Source of Water as a Product
Acid-base neutralization reactions are a classic example of synthesis where water is always a product. These reactions involve the reaction between an acid and a base, producing salt and water. The general form of an acid-base neutralization reaction is:
Acid + Base → Salt + Water
For instance, the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) yields sodium chloride (NaCl) and water (H₂O):
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
Here, the hydrogen ion (H⁺) from the acid combines with the hydroxide ion (OH⁻) from the base to form water. The remaining ions, sodium (Na⁺) and chloride (Cl⁻), form the salt, sodium chloride. This reaction is exothermic, meaning it releases heat. Many other strong acid-strong base reactions follow this pattern, consistently producing water as a product.
Examples of Acid-Base Neutralization Reactions Producing Water:
- Sulfuric acid and potassium hydroxide: H₂SO₄(aq) + 2KOH(aq) → K₂SO₄(aq) + 2H₂O(l)
- Nitric acid and calcium hydroxide: 2HNO₃(aq) + Ca(OH)₂(aq) → Ca(NO₃)₂(aq) + 2H₂O(l)
- Phosphoric acid and ammonia: H₃PO₄(aq) + 3NH₃(aq) → (NH₄)₃PO₄(aq) + 3H₂O(l)
Notice that the number of water molecules produced depends on the stoichiometry of the reaction, dictated by the number of acidic protons (H⁺) and hydroxide ions (OH⁻) involved.
Other Synthesis Reactions Producing Water:
While acid-base neutralizations are the most common, water can also be formed in other synthesis reactions. These reactions might involve the reaction of metal oxides with water or the combustion of hydrocarbons under certain conditions, resulting in water as one of the products.
-
Reaction of a metal oxide with water: Some metal oxides react with water to form metal hydroxides. For example, the reaction of sodium oxide with water yields sodium hydroxide:
Na₂O(s) + H₂O(l) → 2NaOH(aq)
-
Combustion of hydrocarbons: The complete combustion of hydrocarbons (compounds containing carbon and hydrogen) in the presence of sufficient oxygen produces carbon dioxide and water. This is a crucial reaction in energy production and is responsible for much of the water vapor in the atmosphere:
CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(g) (Methane combustion)
These are just two examples. Many other organic and inorganic reactions can lead to the formation of water as a byproduct during synthesis.
Understanding Decomposition Reactions: Water as a Reactant
In contrast to synthesis, decomposition reactions involve the breakdown of a single compound into two or more simpler substances. Here, water often acts as a reactant participating in the decomposition process. This is particularly evident in hydrolysis reactions.
Hydrolysis Reactions: Breaking Down Compounds with Water
Hydrolysis reactions are decomposition reactions where water is a reactant, splitting a molecule into two or more smaller molecules by the addition of a water molecule. This is common in the breakdown of salts, esters, and other compounds.
Hydrolysis of Salts:
Some salts undergo hydrolysis in water, resulting in the formation of an acidic or basic solution. For example, the hydrolysis of sodium acetate produces acetic acid and sodium hydroxide:
CH₃COONa(aq) + H₂O(l) → CH₃COOH(aq) + NaOH(aq)
Hydrolysis of Esters:
Esters, which are commonly found in fats and oils, undergo hydrolysis in the presence of an acid or base catalyst. This process breaks down the ester into its constituent carboxylic acid and alcohol.
RCOOR'(l) + H₂O(l) → RCOOH(aq) + R'OH(aq)
Where R and R' represent alkyl or aryl groups. This reaction is fundamental in the digestion of fats and oils in our bodies.
Other Decomposition Reactions Involving Water:
While hydrolysis is the most common example of decomposition reactions involving water, other reactions exist. For instance, dehydration reactions involve removing water molecules from a compound, often leading to the formation of a more complex molecule. However, the focus here is on reactions where water participates as a reactant in the decomposition process.
Distinguishing Synthesis and Decomposition Reactions
It's crucial to understand the key differences between synthesis and decomposition reactions to correctly identify when water is a product or a reactant:
| Feature | Synthesis Reaction | Decomposition Reaction |
|---|---|---|
| Reactants | Two or more simpler substances | One complex substance |
| Products | One more complex substance | Two or more simpler substances |
| Water's Role | Often a product (especially in acid-base reactions) | Often a reactant (in hydrolysis reactions) |
| Energy Change | Often exothermic (releases heat) | Often endothermic (absorbs heat) |
Conclusion: The Versatility of Water in Chemical Reactions
Water's role in chemical reactions extends beyond simply being a solvent. It acts as both a reactant and a product in a wide variety of processes. Understanding the types of reactions where water is consistently formed – primarily acid-base neutralization reactions and other synthesis reactions – is essential for comprehending many fundamental chemical concepts. Equally important is recognizing situations where water acts as a reactant, such as in hydrolysis reactions, leading to the breakdown of complex molecules. Mastering these concepts provides a solid foundation for further explorations in chemistry. The multifaceted nature of water underscores its significance in the natural world and its impact on numerous chemical processes.
Latest Posts
Latest Posts
-
A Person Pushing A Horizontal Uniformly Loaded
Mar 12, 2025
-
Pursuing A Strategy Of Social Responsibility And Corporate Citizenship
Mar 12, 2025
-
Lokes Was Thrilled When She Found A Low Cost Airfare
Mar 12, 2025
-
Match The Description With The Appropriate Business Process Terms
Mar 12, 2025
-
Question Corndog Draw The Skeletal Structure
Mar 12, 2025
Related Post
Thank you for visiting our website which covers about Water Is Always A Product In What Type Of Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
