Urea Is An Organic Compound Widely Used As A Fertilizer
Holbox
Mar 26, 2025 · 6 min read
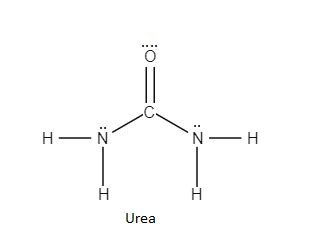
Table of Contents
- Urea Is An Organic Compound Widely Used As A Fertilizer
- Table of Contents
- Urea: The Cornerstone of Modern Agriculture
- What is Urea?
- The Discovery and Synthesis of Urea: A Milestone in Chemistry
- The Industrial Production of Urea
- 1. Ammonia Production: The Foundation
- 2. Urea Synthesis: A Two-Step Process
- 3. Purification and Granulation: Improving the Product
- Applications of Urea in Agriculture
- 1. Soil Application: Direct and Efficient
- 2. Foliar Application: Quick Nutrient Delivery
- 3. Specialized Applications: Beyond General Fertilization
- Environmental Considerations of Urea Use
- 1. Ammonia Volatilization: A Significant Nitrogen Loss
- 2. Nitrate Leaching: Groundwater Contamination
- 3. Eutrophication: Water Body Degradation
- Urea: Future Trends and Innovations
- 1. Urease Inhibitors: Enhancing Nitrogen Use Efficiency
- 2. Controlled-Release Technologies: Optimizing Nutrient Availability
- 3. Precision Agriculture: Targeted Fertilizer Application
- Conclusion: Urea's Enduring Role in Agriculture
- Latest Posts
- Latest Posts
- Related Post
Urea: The Cornerstone of Modern Agriculture
Urea, a simple yet remarkably versatile organic compound, plays a pivotal role in modern agriculture. Its widespread use as a nitrogen-rich fertilizer has revolutionized food production, enabling farmers to cultivate higher yields and feed a burgeoning global population. Understanding urea's properties, production methods, applications, and environmental impact is crucial for anyone involved in agriculture, environmental science, or chemical engineering. This comprehensive article delves into the multifaceted world of urea, exploring its significance from the molecular level to its global implications.
What is Urea?
Urea, chemically known as carbamide, is an organic compound with the formula (NH₂)₂CO. It's a colorless, crystalline solid that's highly soluble in water. What makes urea unique is its exceptionally high nitrogen content (46%), making it an incredibly efficient nitrogen fertilizer. Plants require nitrogen for healthy growth, particularly for chlorophyll production and protein synthesis. Urea's high nitrogen concentration minimizes the amount needed for effective fertilization, reducing transportation and storage costs.
The Discovery and Synthesis of Urea: A Milestone in Chemistry
The discovery of urea holds a significant place in the history of chemistry. In 1828, Friedrich Wöhler famously synthesized urea in a laboratory setting from inorganic ammonium cyanate. This groundbreaking achievement shattered the prevailing vitalist theory, which posited that organic compounds could only be produced by living organisms. Wöhler's synthesis demonstrated that organic molecules could be created artificially, opening new avenues in organic chemistry and paving the way for the industrial production of urea.
The Industrial Production of Urea
The industrial production of urea is a significant chemical process that utilizes readily available raw materials and advanced technologies. The primary raw materials are ammonia (NH₃) and carbon dioxide (CO₂). The process generally follows these steps:
1. Ammonia Production: The Foundation
Ammonia production, primarily through the Haber-Bosch process, is the first critical step. This process involves reacting nitrogen from the air with hydrogen under high pressure and temperature in the presence of a catalyst. The resulting ammonia forms the backbone of urea synthesis.
2. Urea Synthesis: A Two-Step Process
The actual urea synthesis happens in two main steps:
-
Carbamate Formation: Ammonia and carbon dioxide react under high pressure (140-200 atm) and temperature (180-200°C) to form ammonium carbamate (NH₂COONH₄). This is an equilibrium reaction, meaning it proceeds in both directions simultaneously.
-
Dehydration to Urea: The ammonium carbamate then undergoes dehydration, losing a water molecule to form urea. This reaction is also influenced by temperature and pressure, with optimal conditions leading to higher urea yields.
3. Purification and Granulation: Improving the Product
After synthesis, the urea solution undergoes purification to remove impurities and unwanted byproducts. This often involves crystallization, followed by drying and granulation to produce the granular urea commonly used in agriculture. Granulation improves the handling and application of the fertilizer, preventing dust formation and ensuring even distribution.
Applications of Urea in Agriculture
Urea’s dominant application lies in agriculture, where it serves as a primary source of nitrogen for various crops. Its high nitrogen concentration and solubility make it an ideal choice for:
1. Soil Application: Direct and Efficient
Urea can be applied directly to the soil, either as a granular fertilizer or in solution. Once applied, urea undergoes hydrolysis, breaking down into ammonium and bicarbonate ions. These ions are subsequently converted by soil microorganisms into other forms of nitrogen available for plant uptake. The timing and method of application are crucial to minimize nitrogen loss through volatilization (loss to the atmosphere as ammonia) and leaching (movement of nitrogen through the soil profile).
2. Foliar Application: Quick Nutrient Delivery
Urea can also be applied directly to plant leaves (foliar application). This method provides a rapid source of nitrogen, particularly beneficial during periods of high growth demand. However, foliar application requires careful consideration of factors like concentration, timing, and weather conditions to prevent leaf burn or nutrient runoff.
3. Specialized Applications: Beyond General Fertilization
Urea's versatility extends beyond basic fertilization. It’s used in:
- Controlled-release fertilizers: Urea is incorporated into slow-release formulations that gradually release nitrogen over time, optimizing nutrient availability and minimizing environmental losses.
- Starter fertilizers: Urea can be used as a component of starter fertilizers, providing an immediate boost to young seedlings.
- Hydroponics and soilless culture: Urea is a vital component of nutrient solutions used in hydroponic systems and soilless cultivation.
Environmental Considerations of Urea Use
While urea is highly effective as a fertilizer, its use is not without environmental consequences. Understanding and mitigating these impacts is crucial for sustainable agriculture:
1. Ammonia Volatilization: A Significant Nitrogen Loss
The conversion of urea to ammonia is a significant source of nitrogen loss. Ammonia is a potent greenhouse gas, contributing to climate change. Volatilization is particularly problematic under alkaline soil conditions and high temperatures. Techniques like incorporating urea into the soil, using urease inhibitors (substances that slow down urea breakdown), and applying urea at the appropriate time can help minimize volatilization.
2. Nitrate Leaching: Groundwater Contamination
Urea's rapid hydrolysis can lead to the formation of nitrate ions (NO₃⁻), which are highly mobile in soil. Nitrate can leach into groundwater, contaminating drinking water sources and potentially causing health problems (such as methemoglobinemia). Efficient irrigation practices, balanced fertilization, and the selection of suitable crops can help mitigate nitrate leaching.
3. Eutrophication: Water Body Degradation
Runoff from agricultural fields containing urea can lead to eutrophication in surface waters. Excessive nitrogen enrichment fuels excessive algal growth, reducing oxygen levels and harming aquatic life. Proper land management, buffer strips, and integrated nutrient management strategies can minimize eutrophication.
Urea: Future Trends and Innovations
Research and development continue to focus on improving urea's efficiency and minimizing its environmental footprint. Some key advancements include:
1. Urease Inhibitors: Enhancing Nitrogen Use Efficiency
Urease inhibitors slow down the enzymatic breakdown of urea, reducing ammonia volatilization and increasing nitrogen use efficiency. These inhibitors are incorporated into fertilizer formulations, improving the overall performance and sustainability of urea-based fertilizers.
2. Controlled-Release Technologies: Optimizing Nutrient Availability
Advancements in controlled-release technologies provide more precise control over nitrogen release, optimizing nutrient availability for plant uptake and minimizing nitrogen losses. These technologies are crucial for improving fertilizer use efficiency and reducing environmental impacts.
3. Precision Agriculture: Targeted Fertilizer Application
Precision agriculture techniques, such as variable rate application and sensor-based monitoring, enable farmers to tailor fertilizer application to the specific needs of individual fields. This approach optimizes fertilizer use, minimizes waste, and reduces environmental impacts.
Conclusion: Urea's Enduring Role in Agriculture
Urea remains a cornerstone of modern agriculture, providing a highly efficient and cost-effective source of nitrogen for food production. Its widespread use has significantly enhanced crop yields, enabling farmers to meet the growing global demand for food. However, it’s essential to recognize and address the environmental challenges associated with urea use. Ongoing research, innovation, and responsible agricultural practices are crucial for ensuring that urea continues to contribute to food security while minimizing its impact on the environment. The future of urea in agriculture will likely involve a greater emphasis on sustainable practices, precision application, and innovative technologies to optimize its use and minimize its environmental footprint. Continued research and development in areas like urease inhibitors, controlled-release technologies, and precision agriculture will play a vital role in shaping this future and ensuring the responsible and sustainable utilization of urea in the years to come.
Latest Posts
Latest Posts
-
A 5 Month Pregnant Patient Arrives In The Emergency Department
Mar 30, 2025
-
Which Two Items Are Closely Related To The Reserve Requirement
Mar 30, 2025
-
Use The Table To Answer The Questions
Mar 30, 2025
-
What Multiplies To 12 And Adds To
Mar 30, 2025
-
There Are Three Industrial Firms In Happy Valley
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Urea Is An Organic Compound Widely Used As A Fertilizer . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
