To Draw A Lewis Structure One Must Know The
Holbox
Mar 30, 2025 · 6 min read
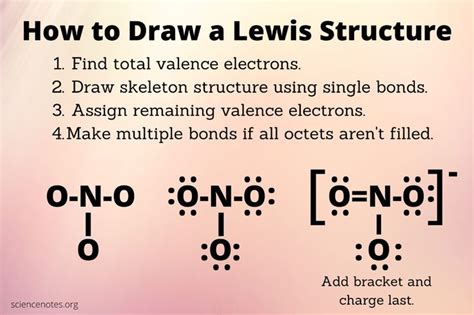
Table of Contents
- To Draw A Lewis Structure One Must Know The
- Table of Contents
- To Draw a Lewis Structure, One Must Know the Fundamentals
- 1. Understanding Valence Electrons: The Building Blocks of Lewis Structures
- Determining Valence Electrons:
- 2. Recognizing Octet Rule and its Exceptions: The Stability Goal
- Exceptions to the Octet Rule:
- 3. Identifying the Central Atom: The Structural Foundation
- 4. Counting Total Valence Electrons: The Electron Inventory
- 5. Drawing Single Bonds and Placing Remaining Electrons: The Structural Framework
- 6. Checking for Octet Rule Satisfaction and Formal Charges: The Validation Process
- Formal Charge Calculation:
- 7. Resonance Structures: Representing Delocalized Electrons
- 8. Practice Makes Perfect: Mastering the Skill
- Advanced Topics in Lewis Structure Drawing:
- Shapes and Molecular Geometry:
- Polarity and Dipole Moments:
- Applications of Lewis Structures:
- Latest Posts
- Latest Posts
- Related Post
To Draw a Lewis Structure, One Must Know the Fundamentals
Drawing Lewis structures, also known as Lewis dot diagrams, is a fundamental skill in chemistry. These diagrams visually represent the valence electrons of atoms in a molecule, helping us understand bonding, molecular geometry, and predict properties. But before you can master the art of drawing Lewis structures, you need a solid understanding of several key concepts. This comprehensive guide will delve into those prerequisites, providing a step-by-step approach to confidently creating accurate Lewis structures.
1. Understanding Valence Electrons: The Building Blocks of Lewis Structures
The cornerstone of any Lewis structure is the valence electron. These are the electrons located in the outermost shell (energy level) of an atom. They are the electrons actively involved in chemical bonding and determine the atom's reactivity. Knowing how many valence electrons an atom possesses is absolutely crucial.
Determining Valence Electrons:
The number of valence electrons is directly related to an atom's position on the periodic table. Specifically:
- Groups 1A and 2A (alkali and alkaline earth metals): These groups have 1 and 2 valence electrons, respectively.
- Groups 3A to 8A (main group elements): The group number (using the older numbering system) directly corresponds to the number of valence electrons. For example, Group 4A elements (like carbon) have 4 valence electrons, while Group 7A elements (halogens) have 7.
- Transition metals: These elements have more complex valence electron configurations, making their Lewis structures more challenging and often require consideration of oxidation states. We will focus primarily on main group elements in this guide.
Example: Oxygen (O) is in Group 6A, so it has 6 valence electrons. Carbon (C) is in Group 4A, so it has 4 valence electrons.
2. Recognizing Octet Rule and its Exceptions: The Stability Goal
The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable electron configuration with eight electrons in their outermost shell, similar to the noble gases. This stable configuration minimizes energy. While a powerful guiding principle, it's crucial to understand its limitations.
Exceptions to the Octet Rule:
Not all atoms strictly follow the octet rule. Common exceptions include:
- Incomplete octet: Some atoms, particularly those in the second period (like boron and beryllium), can have fewer than eight electrons in their valence shell.
- Expanded octet: Atoms in the third period and beyond (elements with d-orbitals available) can accommodate more than eight valence electrons, often forming hypervalent compounds.
- Odd-electron molecules (free radicals): Molecules with an odd number of total valence electrons cannot have all atoms obeying the octet rule.
Example: Boron trifluoride (BF₃) has an incomplete octet for boron, while sulfur hexafluoride (SF₆) exemplifies an expanded octet for sulfur.
3. Identifying the Central Atom: The Structural Foundation
In most molecules, there's a central atom around which the other atoms are arranged. Identifying the central atom is a key step in constructing the Lewis structure. Generally, the least electronegative atom (the atom that holds onto electrons less tightly) acts as the central atom. However, hydrogen (H) and halogens (F, Cl, Br, I) rarely act as central atoms.
Example: In methane (CH₄), carbon is the central atom because it's less electronegative than hydrogen. In carbon dioxide (CO₂), carbon is the central atom.
4. Counting Total Valence Electrons: The Electron Inventory
Before drawing bonds, accurately determine the total number of valence electrons available in the molecule or ion. This is done by summing the valence electrons of each atom. Remember to adjust for the charge of any ions:
- Anions (negative ions): Add one electron for each negative charge.
- Cations (positive ions): Subtract one electron for each positive charge.
Example: For the sulfate ion (SO₄²⁻), we add the valence electrons of sulfur (6) and four oxygens (4 x 6 = 24), then add two more electrons for the 2- charge, giving a total of 32 valence electrons.
5. Drawing Single Bonds and Placing Remaining Electrons: The Structural Framework
Once you've determined the total valence electrons, start by forming single bonds between the central atom and the surrounding atoms. Each single bond consists of two electrons (one electron pair).
After forming the single bonds, distribute the remaining electrons as lone pairs around the atoms to satisfy the octet rule (or its exceptions, as noted above). Begin by placing lone pairs around the outer atoms to fulfill their octets, then place any remaining electrons on the central atom.
6. Checking for Octet Rule Satisfaction and Formal Charges: The Validation Process
After distributing all the electrons, check if all atoms (except for exceptions) satisfy the octet rule. If atoms lack octets, you may need to convert lone pairs into bonding pairs to form double or triple bonds.
Formal Charge Calculation:
Formal charge helps determine the most likely Lewis structure among multiple possibilities. It's calculated as:
Formal Charge = (Valence electrons) - (Non-bonding electrons) - (1/2 Bonding electrons)
A lower formal charge on atoms is generally preferred.
7. Resonance Structures: Representing Delocalized Electrons
Some molecules exhibit resonance, meaning that the electron distribution can't be represented by a single Lewis structure. In these cases, multiple resonance structures are drawn, showing the different possible locations of double or triple bonds. The actual molecule is a hybrid of these resonance structures. The true structure is an average of all resonance structures, with electrons being delocalized (spread out) across multiple atoms.
Example: Ozone (O₃) has two resonance structures to represent the delocalization of the double bond.
8. Practice Makes Perfect: Mastering the Skill
Drawing Lewis structures is a skill that improves with practice. Start with simple molecules and gradually work your way towards more complex ones. Don't be afraid to make mistakes; they're valuable learning opportunities. Work through numerous examples, paying close attention to the steps outlined above.
Advanced Topics in Lewis Structure Drawing:
Shapes and Molecular Geometry:
Once you have a Lewis structure, you can predict the shape of the molecule using concepts like VSEPR (Valence Shell Electron Pair Repulsion) theory. Lone pairs of electrons and bonding pairs repel each other, influencing the overall molecular geometry.
Polarity and Dipole Moments:
The Lewis structure helps determine the polarity of bonds and the overall molecule. Electronegativity differences between atoms determine bond polarity. The arrangement of polar bonds influences the overall dipole moment of the molecule.
Applications of Lewis Structures:
Lewis structures are invaluable tools for various chemical applications:
- Predicting Reactivity: Understanding the distribution of electrons helps predict how a molecule will react with other substances.
- Understanding Bonding: Lewis structures clarify the types of bonds (single, double, triple) and their strengths.
- Predicting Properties: Structural information from Lewis structures correlates with various physical and chemical properties.
In summary, drawing a Lewis structure requires a strong grasp of valence electrons, the octet rule (and its exceptions), the ability to identify central atoms, accurate electron counting, and the principles of formal charge and resonance. While mastering this skill may require time and effort, the ability to visualize and interpret molecular structure is crucial for a deeper understanding of chemistry. Consistent practice, coupled with a solid understanding of the underlying principles, will ultimately lead to proficiency in drawing and interpreting Lewis structures.
Latest Posts
Latest Posts
-
A Processing Department Is An Organization Unit
Apr 01, 2025
-
Which Phenomenon Is Reduced By Oil Immersion Microscopy
Apr 01, 2025
-
A Liability For Cash Dividends Is Recorded
Apr 01, 2025
-
You Can Recognize The Process Of Pinocytosis When
Apr 01, 2025
-
Draw The Correct Product For The Given Diels Alder Reaction
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about To Draw A Lewis Structure One Must Know The . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
