The Graph Shows The Oxygen-binding Curves For Myoglobin And Hemoglobin
Holbox
Mar 12, 2025 · 6 min read
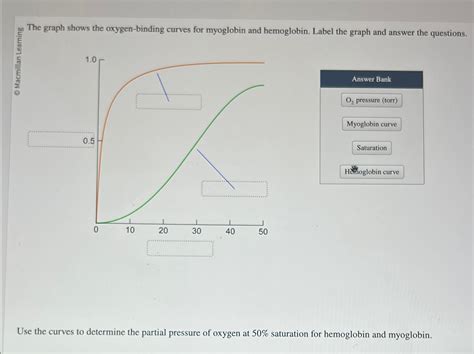
Table of Contents
The Graph Shows the Oxygen-Binding Curves for Myoglobin and Hemoglobin: A Deep Dive into Muscle Function and Oxygen Transport
The oxygen-binding curves for myoglobin and hemoglobin are fundamental to understanding how oxygen is transported and utilized in the body. These proteins, though structurally similar, exhibit distinct binding properties that are crucial for their respective roles. This article will delve into the specifics of these curves, exploring their shapes, the underlying biochemical mechanisms, and the physiological implications of their differences.
Understanding Myoglobin and Hemoglobin: Structure and Function
Before diving into the curves, it's essential to understand the proteins themselves. Both myoglobin and hemoglobin are hemeproteins, meaning they contain a heme prosthetic group—a porphyrin ring complexed with an iron ion (Fe<sup>2+</sup>)—that binds oxygen reversibly. However, their structures and functions differ significantly:
Myoglobin: The Muscle Oxygen Store
Myoglobin is a monomeric protein found predominantly in the muscle tissue of vertebrates. Its primary function is oxygen storage. It acts as a reservoir, releasing oxygen to the mitochondria during periods of high metabolic demand, such as intense muscle activity. Its high affinity for oxygen ensures that it readily binds oxygen when available and releases it only when oxygen partial pressure (pO<sub>2</sub>) is very low.
Hemoglobin: The Oxygen Transporter
Hemoglobin, on the other hand, is a tetrameric protein (four subunits) found in red blood cells. Its primary function is oxygen transport from the lungs (or gills in aquatic animals) to the tissues. Unlike myoglobin, hemoglobin's oxygen affinity is modulated by several factors, enabling it to efficiently load oxygen in the lungs and unload it in the tissues. This modulation is crucial for efficient oxygen delivery throughout the body.
The Oxygen-Binding Curves: A Visual Representation
The oxygen-binding curves visually depict the relationship between the partial pressure of oxygen (pO<sub>2</sub>) and the fractional saturation (Y) of the protein with oxygen. Fractional saturation refers to the percentage of oxygen-binding sites occupied by oxygen molecules.
Myoglobin's Hyperbolic Curve
Myoglobin's oxygen-binding curve is hyperbolic. This shape reflects its high affinity for oxygen. At relatively low pO<sub>2</sub>, myoglobin readily binds oxygen, reaching near-saturation even at low oxygen partial pressures. This characteristic is ideal for its role as an oxygen store, readily binding oxygen even when the oxygen levels in the muscle are relatively low. The hyperbolic shape indicates that the binding of oxygen to one myoglobin molecule doesn't significantly influence the binding of oxygen to other molecules (since it's a monomer).
Hemoglobin's Sigmoidal Curve
Hemoglobin's oxygen-binding curve, in contrast, is sigmoidal (S-shaped). This sigmoidal shape is a consequence of cooperativity, a phenomenon where the binding of one oxygen molecule to one subunit influences the oxygen affinity of the other subunits. This cooperative binding allows hemoglobin to efficiently load oxygen in the lungs (high pO<sub>2</sub>) and unload it in the tissues (low pO<sub>2</sub>).
The Significance of the Sigmoidal Curve: Cooperative Binding and Allosteric Regulation
The sigmoidal curve of hemoglobin's oxygen-binding reflects its crucial allosteric properties. Allosteric regulation refers to the modulation of protein function by binding of a molecule at a site other than the active site. In hemoglobin, the binding of oxygen to one subunit induces conformational changes that increase the oxygen affinity of the remaining subunits. This cooperative binding is essential for efficient oxygen transport:
-
High pO<sub>2</sub> (Lungs): In the lungs, where pO<sub>2</sub> is high, oxygen readily binds to one subunit, triggering conformational changes that facilitate the binding of oxygen to the other subunits. This leads to near-saturation of hemoglobin with oxygen.
-
Low pO<sub>2</sub> (Tissues): In the tissues, where pO<sub>2</sub> is low, the release of one oxygen molecule from a subunit induces conformational changes that reduce the oxygen affinity of the other subunits. This facilitates the release of more oxygen molecules, ensuring efficient oxygen delivery to the tissues.
Factors Affecting Hemoglobin's Oxygen Affinity: The Bohr Effect and 2,3-Bisphosphoglycerate (2,3-BPG)
Hemoglobin's oxygen affinity is not solely determined by pO<sub>2</sub>. Several factors modulate its binding capacity, further enhancing its efficiency as an oxygen transporter:
The Bohr Effect: pH and Carbon Dioxide
The Bohr effect describes the influence of pH and carbon dioxide on hemoglobin's oxygen affinity. A decrease in pH (increased acidity) or an increase in carbon dioxide concentration reduces hemoglobin's oxygen affinity, facilitating oxygen unloading in metabolically active tissues. These tissues produce more CO<sub>2</sub> and lactic acid, lowering pH and thus promoting oxygen release.
2,3-Bisphosphoglycerate (2,3-BPG): A Crucial Regulator
2,3-BPG is a small molecule found in red blood cells that binds to hemoglobin, reducing its oxygen affinity. This effect is particularly important at high altitudes or during strenuous exercise, where oxygen levels may be low. By reducing hemoglobin's affinity, 2,3-BPG ensures that a greater proportion of oxygen is released to the tissues, even at lower pO<sub>2</sub> levels.
Comparing Myoglobin and Hemoglobin: A Summary
| Feature | Myoglobin | Hemoglobin |
|---|---|---|
| Structure | Monomeric | Tetrameric |
| Function | Oxygen storage | Oxygen transport |
| Oxygen Affinity | High | Lower (modulated) |
| Curve Shape | Hyperbolic | Sigmoidal |
| Cooperativity | Absent | Present |
| Allosteric Regulation | Minimal | Significant (Bohr effect, 2,3-BPG) |
Clinical Significance and Further Research
Understanding the oxygen-binding curves of myoglobin and hemoglobin is crucial in various medical contexts. For instance, analyzing the shape and position of these curves can aid in diagnosing certain conditions affecting oxygen transport, such as anemia or carbon monoxide poisoning. Furthermore, research continues to explore the intricate details of hemoglobin's allosteric regulation, aiming to develop therapeutic strategies for improving oxygen delivery in patients with respiratory or circulatory disorders. Understanding the nuances of oxygen binding, therefore, is not only a fundamental aspect of physiology but also holds significant translational potential for medical advancements.
Conclusion
The distinct oxygen-binding curves of myoglobin and hemoglobin reflect their specialized roles in oxygen storage and transport. Myoglobin's hyperbolic curve reflects its high oxygen affinity, making it an efficient oxygen reservoir in muscle tissue. Hemoglobin's sigmoidal curve, a result of cooperative binding and allosteric regulation, ensures efficient oxygen loading in the lungs and unloading in the tissues. This intricate interplay of structure, function, and regulation highlights the remarkable adaptability of these proteins in supporting life's essential processes. Further research into the complexities of these curves will undoubtedly continue to illuminate our understanding of physiological function and disease. This deeper understanding can lead to improved diagnostic tools and therapeutic interventions for a range of health conditions impacting oxygen delivery and utilization.
Latest Posts
Latest Posts
-
Locking Out Tagging Out Refers To The Practice Of
Mar 12, 2025
-
What Is The Value Of The
Mar 12, 2025
-
You Open A Document To Find The Text Illegible
Mar 12, 2025
-
On July 1 A Company Receives An Invoice For 800
Mar 12, 2025
-
Match Each Term With Its Definition
Mar 12, 2025
Related Post
Thank you for visiting our website which covers about The Graph Shows The Oxygen-binding Curves For Myoglobin And Hemoglobin . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
