The Electrophilic Aromatic Substitution Of Isopropylbenzene With Br2 And Febr3
Holbox
Mar 27, 2025 · 6 min read
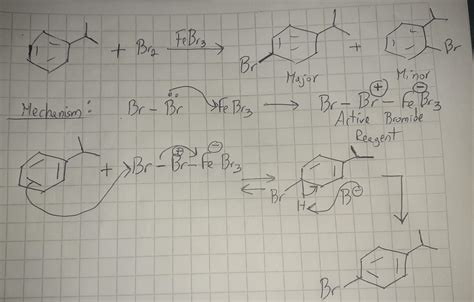
Table of Contents
- The Electrophilic Aromatic Substitution Of Isopropylbenzene With Br2 And Febr3
- Table of Contents
- Electrophilic Aromatic Substitution of Isopropylbenzene with Br₂ and FeBr₃: A Deep Dive
- Understanding Electrophilic Aromatic Substitution
- 1. Generation of the Electrophile
- 2. Attack of the Electrophile and Formation of the Arenium Ion
- 3. Deprotonation and Regeneration of Aromaticity
- Bromination of Isopropylbenzene (Cumene): Regioselectivity
- Reaction Mechanism: A Detailed Look
- Practical Considerations and Experimental Setup
- Step-by-Step Procedure (Conceptual):
- Applications and Significance
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Electrophilic Aromatic Substitution of Isopropylbenzene with Br₂ and FeBr₃: A Deep Dive
The electrophilic aromatic substitution (EAS) reaction is a cornerstone of organic chemistry, allowing for the introduction of various electrophiles onto aromatic rings. This article will delve into the specific case of the bromination of isopropylbenzene (cumene) using bromine (Br₂) and iron(III) bromide (FeBr₃) as a catalyst. We will explore the reaction mechanism, the regioselectivity observed, and the practical considerations involved in this important transformation.
Understanding Electrophilic Aromatic Substitution
Before diving into the specifics of cumene bromination, let's review the fundamental principles of EAS reactions. These reactions involve the attack of an electrophile (an electron-deficient species) on the electron-rich aromatic ring. The aromatic ring, despite its stability due to resonance, is susceptible to attack by strong electrophiles because of the high electron density delocalized across the pi system. The reaction proceeds through a series of steps:
1. Generation of the Electrophile
The key to a successful EAS reaction is the generation of a potent electrophile. In the case of bromination using Br₂ and FeBr₃, the catalyst plays a crucial role. FeBr₃ acts as a Lewis acid, coordinating with Br₂ to polarize the bromine-bromine bond. This polarization significantly increases the electrophilicity of bromine, making it a much better electrophile. The reaction can be represented as follows:
Br₂ + FeBr₃ ⇌ Br⁺ + FeBr₄⁻
This generates a bromonium ion (Br⁺), a highly reactive electrophile, which is ready to attack the aromatic ring.
2. Attack of the Electrophile and Formation of the Arenium Ion
The bromonium ion then attacks the electron-rich pi system of the aromatic ring. This attack results in the formation of a resonance-stabilized carbocation intermediate, also known as an arenium ion or sigma complex. The positive charge is delocalized across the ring, lessening the overall positive charge density. This step is usually the rate-determining step of the reaction.
3. Deprotonation and Regeneration of Aromaticity
The arenium ion is not aromatic because it lacks the 4n+2 pi electrons characteristic of aromatic systems. To regain aromaticity, a proton (H⁺) is abstracted from the arenium ion by the FeBr₄⁻ anion (or another suitable base). This step restores the aromatic system and generates the brominated product.
Bromination of Isopropylbenzene (Cumene): Regioselectivity
The bromination of isopropylbenzene is not simply a matter of adding a bromine atom to the ring; it's also crucial to consider where the bromine atom will be positioned. Isopropylbenzene contains an isopropyl group, which is an alkyl substituent. Alkyl groups are electron-donating groups (EDGs). EDGs activate the aromatic ring towards electrophilic attack and direct the incoming electrophile to specific positions on the ring.
The isopropyl group is an ortho/para-directing group. This means that the incoming bromine atom will preferentially substitute at the ortho (adjacent) or para (opposite) positions relative to the isopropyl group. This is due to the resonance stabilization of the arenium ion intermediates formed when the electrophile attacks at these positions. The ortho and para positions allow the positive charge in the arenium ion to be delocalized closer to the electron-donating isopropyl group, resulting in more stable intermediates.
Therefore, the major products of the reaction are 1-bromo-2-isopropylbenzene (ortho isomer) and 1-bromo-4-isopropylbenzene (para isomer). The meta isomer (1-bromo-3-isopropylbenzene) is formed in much smaller quantities, if at all.
Reaction Mechanism: A Detailed Look
Let's illustrate the reaction mechanism with detailed step-by-step diagrams, showing the formation of both the ortho and para isomers:
(Diagram 1: Electrophile Generation - already described above)
(Diagram 2: Ortho Attack)
- Attack: The bromonium ion attacks the ortho position of the isopropylbenzene ring.
- Arenium Ion Formation: A resonance-stabilized arenium ion is formed. The positive charge is delocalized across the ring, with significant contribution from the carbon atoms adjacent to the isopropyl group.
- Deprotonation: The FeBr₄⁻ anion abstracts a proton from the arenium ion, regenerating aromaticity and forming 1-bromo-2-isopropylbenzene.
(Diagram 3: Para Attack)
- Attack: The bromonium ion attacks the para position of the isopropylbenzene ring.
- Arenium Ion Formation: A resonance-stabilized arenium ion is formed. Again, the positive charge is delocalized, with contribution from the carbon atoms adjacent to the isopropyl group.
- Deprotonation: The FeBr₄⁻ anion abstracts a proton from the arenium ion, regenerating aromaticity and forming 1-bromo-4-isopropylbenzene.
(Note: Detailed diagrams would require a chemical drawing tool, which is not available in this text-based format. However, visualizing these steps using standard organic chemistry drawing conventions is strongly recommended for a deeper understanding.)
Practical Considerations and Experimental Setup
The bromination of isopropylbenzene is typically carried out under anhydrous conditions to prevent unwanted side reactions. The reaction is usually performed at low to moderate temperatures to control the reaction rate and minimize the formation of byproducts. The use of excess bromine should be avoided to prevent polybromination.
Step-by-Step Procedure (Conceptual):
- Preparation: Isopropylbenzene is dissolved in a suitable anhydrous solvent (e.g., dichloromethane). A catalytic amount of FeBr₃ is added.
- Bromine Addition: A solution of bromine in the same solvent is added dropwise to the reaction mixture. The addition should be slow and controlled to maintain the reaction temperature.
- Reaction Monitoring: The reaction progress can be monitored using techniques like TLC or gas chromatography.
- Workup: Once the reaction is complete, the reaction mixture is quenched with water or aqueous sodium thiosulfate to destroy any unreacted bromine.
- Isolation and Purification: The product mixture can be isolated through extraction and purified using techniques such as distillation or chromatography.
Applications and Significance
The bromination of isopropylbenzene, and EAS reactions in general, hold significant importance in organic synthesis. The resulting brominated products can serve as valuable intermediates for a variety of further transformations, including:
- Grignard Reagent Formation: The aryl bromide can be converted into a Grignard reagent, opening a wide array of carbon-carbon bond-forming reactions.
- Coupling Reactions: The aryl bromide can participate in various coupling reactions, such as Suzuki, Stille, and Sonogashira couplings, to build more complex molecules.
- Nucleophilic Aromatic Substitution: Under specific conditions, the aryl bromide can undergo nucleophilic aromatic substitution, allowing for the introduction of diverse functional groups.
Conclusion
The electrophilic aromatic substitution of isopropylbenzene with Br₂ and FeBr₃ is a classic example of an EAS reaction demonstrating the regioselectivity governed by the directing effects of substituents. Understanding this reaction, including its mechanism and practical considerations, is crucial for organic chemists involved in synthesizing complex molecules. The brominated products serve as versatile intermediates for a wide range of subsequent chemical transformations, highlighting the fundamental importance of EAS in organic synthesis and its contribution to the development of new materials and pharmaceuticals. Further exploration into the kinetics and thermodynamics of this reaction, as well as optimization strategies, will continue to refine our understanding and improve its applicability in various fields.
Latest Posts
Latest Posts
-
Classify Each Of The Following As Acidic Basic Or Neutral
Mar 31, 2025
-
What Three Characteristics Define A Small Business
Mar 31, 2025
-
The Keynesian Economic Framework Is Based On An Assumption That
Mar 31, 2025
-
Socialization As A Sociological Term Describes
Mar 31, 2025
-
The Federal Government Taxes Which Of The Following
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about The Electrophilic Aromatic Substitution Of Isopropylbenzene With Br2 And Febr3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
