Standard Heat Of Formation For H2o
Holbox
Mar 19, 2025 · 6 min read
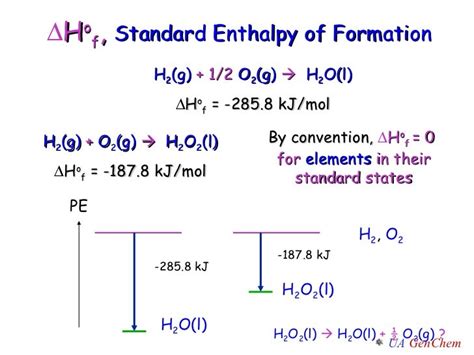
Table of Contents
Standard Heat of Formation for H₂O: A Deep Dive
The standard heat of formation, often denoted as ΔHf°, is a crucial thermodynamic property that represents the change in enthalpy during the formation of one mole of a substance from its constituent elements in their standard states. For water (H₂O), understanding its standard heat of formation is essential in various fields, from chemistry and engineering to environmental science and meteorology. This comprehensive article delves into the intricacies of H₂O's standard heat of formation, exploring its calculation, significance, factors influencing its value, and its applications.
Understanding Standard States and Enthalpy
Before diving into the specifics of H₂O's heat of formation, let's establish a clear understanding of fundamental concepts.
Standard States: The Foundation of Thermodynamic Calculations
The standard state refers to the most stable physical state of a substance at a defined temperature and pressure. For thermodynamic calculations, the standard temperature is usually 298.15 K (25°C) and the standard pressure is 1 atmosphere (101.325 kPa). It's crucial to note that the standard state is not necessarily the most commonly encountered state of a substance under ambient conditions. For instance, while carbon exists as graphite under standard conditions, diamond is also a form of carbon.
Enthalpy: The Heat Content of a System
Enthalpy (H) is a thermodynamic property representing the total heat content of a system at constant pressure. The change in enthalpy (ΔH) during a chemical reaction is the difference between the enthalpy of the products and the enthalpy of the reactants. A negative ΔH indicates an exothermic reaction (heat is released), while a positive ΔH signifies an endothermic reaction (heat is absorbed).
Calculating the Standard Heat of Formation for H₂O
The standard heat of formation for water, H₂O(l) (liquid water), is experimentally determined. The reaction representing its formation is:
H₂(g) + ½O₂(g) → H₂O(l)
This equation depicts the formation of one mole of liquid water from its constituent elements, hydrogen gas (H₂), and oxygen gas (O₂), in their standard states. The standard heat of formation for H₂O(l) is experimentally determined to be approximately -285.8 kJ/mol. The negative sign indicates that this reaction is exothermic; heat is released when water is formed from its elements.
The experimental determination involves techniques like calorimetry, where the heat released or absorbed during a reaction is measured precisely. Sophisticated calorimeters, capable of accurate temperature measurements, are used for this purpose. The heat released is then used to calculate the change in enthalpy, which directly corresponds to the standard heat of formation.
Different Phases, Different Enthalpies
It's important to emphasize that the standard heat of formation varies depending on the phase of water. The value of -285.8 kJ/mol applies specifically to liquid water (H₂O(l)). The standard heat of formation for gaseous water (H₂O(g)) is different, approximately -241.8 kJ/mol. The difference arises from the energy required to change the phase of water from liquid to gas (the heat of vaporization). The formation of gaseous water requires less energy release than liquid water formation, reflecting this difference.
Significance and Applications of H₂O's Heat of Formation
The standard heat of formation of water plays a crucial role in various scientific and engineering disciplines:
1. Thermochemical Calculations:
The standard heat of formation serves as a fundamental building block for calculating the enthalpy changes in numerous other chemical reactions involving water. Hess's Law, a cornerstone of thermochemistry, allows for the calculation of ΔH for any reaction by using the known standard heats of formation of the reactants and products. This enables predictions of whether a reaction will be exothermic or endothermic, facilitating process optimization and design in various industrial applications.
2. Combustion Calculations:
The heat of formation is critical in determining the heat released during combustion reactions. Many combustion processes, such as the burning of fuels, involve the formation of water as a product. By incorporating the heat of formation of water, engineers can accurately estimate the energy output from various fuel sources. This is invaluable in designing efficient power plants, internal combustion engines, and other combustion-based systems.
3. Environmental Science:
In environmental science, the heat of formation plays a role in understanding energy balances in ecosystems. Processes like respiration and photosynthesis involve water, and their energy dynamics are directly linked to the heat of formation. Understanding these energy balances is vital for assessing the environmental impact of various activities and developing strategies for sustainable environmental management.
4. Meteorological Applications:
Meteorology uses the heat of formation of water in modeling weather patterns and climate change. Processes like condensation and evaporation, which involve phase changes of water, release or absorb significant amounts of energy. These energy transfers profoundly influence atmospheric dynamics, and accurate calculations rely on the precise values of the heat of formation.
5. Chemical Engineering:
Chemical engineers utilize the heat of formation in designing and optimizing various industrial processes involving water. Reactions that produce or consume water often have associated heat effects that impact process efficiency and safety. Accurate calculations of these effects are necessary for designing safe and efficient chemical plants.
Factors Influencing the Standard Heat of Formation of Water
Several factors can slightly influence the experimentally determined value of the standard heat of formation for water:
1. Temperature:
The standard heat of formation is temperature-dependent. While the value of -285.8 kJ/mol is for 298.15 K, slight variations occur at other temperatures. This temperature dependence is described by the heat capacity of water, which quantifies how much the enthalpy changes with temperature.
2. Pressure:
Pressure also subtly affects the standard heat of formation. While the standard pressure is 1 atm, deviations from this pressure can introduce small variations. This is particularly relevant at significantly high pressures where the compressibility of water becomes non-negligible.
3. Isotopic Composition:
Water molecules can contain different isotopes of hydrogen (protium, deuterium, tritium) and oxygen. The heat of formation can vary slightly depending on the isotopic composition because the bond energies involving different isotopes are not exactly identical. However, these variations are typically minor for naturally occurring water.
Conclusion: A Foundational Thermodynamic Property
The standard heat of formation for water is a crucial thermodynamic property with far-reaching implications across numerous scientific and engineering disciplines. Its precise determination through calorimetric techniques allows for accurate calculations of enthalpy changes in a wide range of processes. Understanding its value and its dependence on factors like temperature, pressure, and isotopic composition is critical for accurate modeling and prediction in various applications, from chemical engineering to environmental science and meteorology. The negative value consistently highlights the thermodynamic stability of water, which underpins many of Earth's natural processes. As research continues to refine our understanding of thermochemistry, the significance of the heat of formation of water remains steadfast, providing a foundational parameter for a vast array of calculations and predictions.
Latest Posts
Latest Posts
-
Incident Objectives That Drive Incident Operations Are Established By The
Mar 19, 2025
-
Label The Structures Of The Pelvis
Mar 19, 2025
-
Environmental Scanning Is Necessary For An Organization To
Mar 19, 2025
-
Which Of These Are Examples Of Business Analytics
Mar 19, 2025
-
What Statements Are Always True About Limiting Reactants
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Standard Heat Of Formation For H2o . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
