Specific Weight Of Mercury In Lb Ft3
Holbox
Mar 27, 2025 · 5 min read
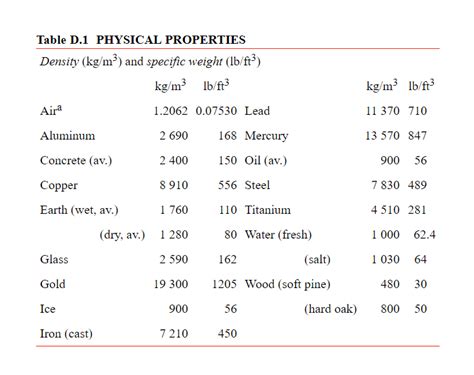
Table of Contents
- Specific Weight Of Mercury In Lb Ft3
- Table of Contents
- Specific Weight of Mercury in lb/ft³: A Comprehensive Guide
- Understanding Specific Weight
- Calculating the Specific Weight of Mercury
- Factors Affecting the Specific Weight of Mercury
- Temperature's Influence
- Pressure's Influence
- Impurities
- Practical Applications of Mercury's Specific Weight
- Engineering Design and Calculations
- Environmental Monitoring
- Scientific Research
- Variations and Considerations
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Specific Weight of Mercury in lb/ft³: A Comprehensive Guide
Mercury, a dense, silvery-white liquid metal, holds significant importance across various scientific and industrial fields. Understanding its properties, especially its specific weight, is crucial for numerous applications, from engineering calculations to environmental monitoring. This comprehensive guide delves into the specific weight of mercury in lb/ft³, exploring its definition, calculation, variations, and practical implications.
Understanding Specific Weight
Before diving into mercury's specific weight, let's clarify the concept. Specific weight, also known as unit weight, represents the weight per unit volume of a substance. It differs from density, which represents mass per unit volume. The relationship between specific weight (γ) and density (ρ) is expressed as:
γ = ρg
Where:
- γ = specific weight (lb/ft³)
- ρ = density (lb/ft³)
- g = acceleration due to gravity (ft/s²)
The acceleration due to gravity (g) varies slightly based on location, but a standard value of 32.174 ft/s² is commonly used. This slight variation needs to be considered for highly precise calculations.
Calculating the Specific Weight of Mercury
The specific weight of mercury is readily calculated using its density. The density of mercury at standard temperature (0°C or 32°F) and pressure is approximately 13,534 kg/m³. To convert this to lb/ft³, we need to employ the following conversion factors:
- 1 kg ≈ 2.20462 lb
- 1 m ≈ 3.28084 ft
Therefore, the conversion proceeds as follows:
13,534 kg/m³ * (2.20462 lb/kg) * (1 m/3.28084 ft)³ ≈ 848.6 lb/ft³
This value represents the approximate specific weight of mercury at standard conditions. It's crucial to remember that this is an approximation, and the actual specific weight can vary slightly based on temperature and pressure.
Factors Affecting the Specific Weight of Mercury
Several factors influence the specific weight of mercury, primarily temperature and pressure. Let's explore these in detail:
Temperature's Influence
Mercury's density, and therefore its specific weight, decreases with increasing temperature. This is because thermal expansion causes the mercury atoms to move further apart, resulting in a decrease in density and consequently, a reduction in specific weight. The relationship between temperature and density is non-linear, and accurate calculations often require the use of empirical equations or tabulated data that account for this non-linearity.
Pressure's Influence
The effect of pressure on mercury's specific weight is relatively minor compared to the influence of temperature. However, at extremely high pressures, the compressibility of mercury becomes noticeable, leading to a slight increase in density and, consequently, specific weight. For most practical applications, the pressure effect can be neglected.
Impurities
The presence of impurities in mercury can also slightly affect its specific weight. Different impurities possess varying densities, leading to deviations from the specific weight of pure mercury. The extent of this variation depends on the type and concentration of impurities. For precise applications, the purity of the mercury used should be carefully considered.
Practical Applications of Mercury's Specific Weight
The specific weight of mercury plays a crucial role in various applications, including:
Engineering Design and Calculations
In engineering, the specific weight of mercury is essential for designing and analyzing systems involving mercury. Examples include:
-
Manometers: Mercury's high density makes it ideal for use in manometers, instruments used to measure pressure differences. The specific weight is directly incorporated into the calculations for determining pressure based on the height of the mercury column.
-
Hydraulic Systems: In specific hydraulic systems employing mercury, its specific weight is critical in calculating forces and pressures within the system.
-
Specific Gravity Determinations: The specific weight of mercury provides a reference point for determining the specific gravity of other substances. By comparing the weight of a substance to the weight of an equal volume of mercury, the specific gravity can be determined.
Environmental Monitoring
Understanding mercury's specific weight is vital for environmental monitoring and remediation efforts:
-
Contamination Assessment: Determining the concentration of mercury in contaminated soil or water often involves measuring its mass or volume. Knowledge of its specific weight is essential for converting measurements between mass and volume, providing an accurate assessment of contamination levels.
-
Spill Response: In the event of a mercury spill, knowing its specific weight aids in predicting its movement and behavior, allowing for more effective containment and cleanup strategies.
Scientific Research
Mercury's properties, particularly its specific weight, are crucial in various scientific research areas, including:
-
Fluid Dynamics: Studies investigating fluid flow and behavior often use mercury as a model fluid. Its high specific weight influences its dynamics and makes it a suitable subject for research.
-
Material Science: The high density and specific weight of mercury are relevant in the study of material properties and interactions.
Variations and Considerations
It's important to emphasize that the specific weight of mercury presented earlier (848.6 lb/ft³) is an approximation at standard conditions. The actual value can vary depending on:
-
Temperature: Always refer to appropriate tables or equations that provide specific weight values as a function of temperature.
-
Pressure: Although generally negligible for most applications, the influence of pressure becomes relevant under extreme conditions.
-
Impurities: The presence of impurities in mercury will affect the precise value of its specific weight. Using high-purity mercury is essential for accurate measurements.
Conclusion
The specific weight of mercury, while seemingly a simple physical property, is a critical parameter in a wide range of scientific, engineering, and environmental applications. Understanding its value and the factors that influence it is crucial for accurate calculations, effective design, and informed decision-making in various fields. Always remember to account for temperature and pressure variations, as well as the potential influence of impurities, for the most accurate determination of mercury's specific weight. Further research and consultation of relevant scientific literature are encouraged for precise applications requiring high accuracy. Always prioritize safety when handling mercury due to its toxicity.
Latest Posts
Latest Posts
-
Health Economics And Policy James Henderson Cite Apa
Mar 30, 2025
-
Which Of The Following Statements About Cycloaddition Reactions Is True
Mar 30, 2025
-
Elisa Graduated From College With A Double Major
Mar 30, 2025
-
The Inflation Rate Is Defined As The
Mar 30, 2025
-
Schedule Of Cost Of Goods Manufactured
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Specific Weight Of Mercury In Lb Ft3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
