Select The Correct Iupac Name For The Cycloalkane
Holbox
Mar 21, 2025 · 5 min read
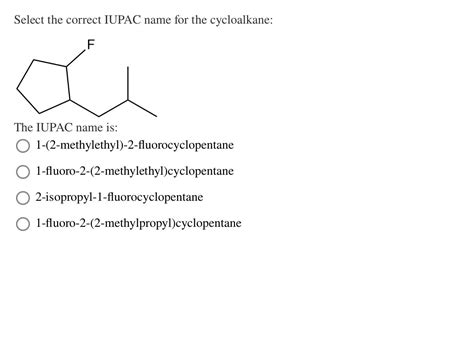
Table of Contents
- Select The Correct Iupac Name For The Cycloalkane
- Table of Contents
- Selecting the Correct IUPAC Name for a Cycloalkane: A Comprehensive Guide
- Understanding the Basics of Cycloalkane Nomenclature
- The Parent Chain: Identifying the Cycloalkane Ring
- Substituents: Identifying Attached Groups
- Numbering the Ring: Establishing the Order of Substituents
- Step-by-Step Guide to Naming Cycloalkanes
- Advanced Cases and Complexities
- Multiple Substituents of the Same Type
- Complex Substituents
- Bicyclic and Polycyclic Systems
- Stereochemistry
- Practical Tips for Success
- Conclusion: Mastering Cycloalkane Nomenclature
- Latest Posts
- Latest Posts
- Related Post
Selecting the Correct IUPAC Name for a Cycloalkane: A Comprehensive Guide
Understanding how to name cycloalkanes correctly is crucial for anyone studying organic chemistry. The International Union of Pure and Applied Chemistry (IUPAC) nomenclature provides a standardized system for naming organic compounds, ensuring clarity and consistency in communication within the scientific community. This comprehensive guide will walk you through the process of selecting the correct IUPAC name for any cycloalkane, covering various complexities and providing numerous examples.
Understanding the Basics of Cycloalkane Nomenclature
Cycloalkanes are saturated cyclic hydrocarbons, meaning they consist of only carbon and hydrogen atoms arranged in a ring structure with single bonds. The fundamental principles of IUPAC nomenclature for cycloalkanes are built upon understanding the parent chain, substituents, and numbering system.
The Parent Chain: Identifying the Cycloalkane Ring
The parent chain is the largest continuous ring of carbon atoms in the molecule. This determines the base name of the cycloalkane. Common cycloalkanes and their IUPAC names include:
- Cyclopropane: A three-membered ring (C₃H₆)
- Cyclobutane: A four-membered ring (C₄H₈)
- Cyclopentane: A five-membered ring (C₅H₁₀)
- Cyclohexane: A six-membered ring (C₆H₁₂)
- Cycloheptane: A seven-membered ring (C₇H₁₄)
- Cyclooctane: An eight-membered ring (C₈H₁₆)
and so on. The names follow a simple pattern: "cyclo" + the alkane name corresponding to the number of carbon atoms in the ring.
Substituents: Identifying Attached Groups
Substituents are atoms or groups of atoms attached to the cycloalkane ring. These are named according to their structure and are listed alphabetically before the parent cycloalkane name. Common substituents include:
- Methyl (CH₃): A single carbon atom attached to three hydrogens.
- Ethyl (CH₂CH₃): A two-carbon chain.
- Propyl (CH₂CH₂CH₃): A three-carbon chain.
- Isopropyl [(CH₃)₂CH]: A branched three-carbon chain.
- Butyl (C₄H₉): A four-carbon chain (various isomers exist).
- And many more...
The number of each type of substituent is indicated using prefixes like di- (two), tri- (three), tetra- (four), penta- (five), and so on.
Numbering the Ring: Establishing the Order of Substituents
Numbering the carbon atoms in the cycloalkane ring is crucial for determining the correct position of the substituents. The numbering should be done in a way that:
- Minimizes the numbers: The substituents should be assigned the lowest possible numbers.
- Prioritizes substituents: Substituents are listed in alphabetical order, ignoring prefixes like di- or tri-.
If multiple numbering schemes give the same lowest numbers, prioritize the substituents alphabetically.
Step-by-Step Guide to Naming Cycloalkanes
Let's break down the process with a detailed example. Consider the following cycloalkane:
! (Imagine a cyclohexane ring with a methyl group on carbon 1 and an ethyl group on carbon 3)
Step 1: Identify the Parent Chain
The largest continuous ring is a six-membered ring, so the parent chain is cyclohexane.
Step 2: Identify and Name the Substituents
We have two substituents:
- Methyl (CH₃)
- Ethyl (CH₂CH₃)
Step 3: Number the Ring
We number the ring carbons to give the lowest possible numbers to the substituents. Starting at the methyl group and going clockwise, we have a methyl group on carbon 1 and an ethyl group on carbon 3. Numbering counter-clockwise would result in the methyl group on carbon 1 and the ethyl group on carbon 5, which uses higher numbers.
Step 4: Arrange the Substituents Alphabetically
The substituents arranged alphabetically are ethyl then methyl.
Step 5: Combine the Information to Form the IUPAC Name
The complete IUPAC name is 3-ethyl-1-methylcyclohexane. Note that the numbers are separated from each other and from the name of the substituent by hyphens.
Advanced Cases and Complexities
The process becomes more complex when dealing with multiple substituents, complex substituents, or rings with different sizes within the molecule. Let's look at some advanced cases:
Multiple Substituents of the Same Type
If a cycloalkane has multiple substituents of the same type, use prefixes like di, tri, tetra, etc., to indicate the number of identical substituents. For example:
-
1,2-dimethylcyclopropane: This molecule has two methyl groups on adjacent carbons of a cyclopropane ring.
-
1,1,2-trimethylcyclopentane: This molecule has three methyl groups; two are on the same carbon and the third is on an adjacent carbon of a cyclopentane ring.
In such cases, the numbering system ensures that the overall number set is the lowest possible.
Complex Substituents
If a substituent is itself a complex molecule with its own substituents, the substituent is named according to its own IUPAC rules. For example, a cyclohexane ring with a propyl group that has a methyl group attached to the second carbon would have the propyl group named as a 2-methylpropyl substituent.
Bicyclic and Polycyclic Systems
These systems involve multiple fused or bridged rings. Naming these becomes considerably more complex and requires the use of specialized nomenclature rules involving bridgehead carbons and numbering systems specific to the ring systems. These are best addressed with dedicated study of bicyclic and polycyclic hydrocarbons.
Stereochemistry
The IUPAC name must also consider the stereochemistry (spatial arrangement of atoms) of the molecule. Cis-trans isomerism (or E/Z isomerism for larger rings) plays a role in determining the complete IUPAC name, especially in cases of disubstituted cycloalkanes. Cis indicates substituents on the same side of the ring plane, while trans indicates substituents on opposite sides.
Practical Tips for Success
-
Practice Regularly: The best way to master cycloalkane nomenclature is to practice. Work through numerous examples, gradually increasing the complexity.
-
Use Systematic Approach: Follow the steps systematically, making sure you address each step thoroughly.
-
Consult Resources: Various textbooks and online resources provide detailed explanations and examples.
-
Verify Your Work: Double-check your work against a reliable source or have it reviewed by someone else to catch any mistakes.
Conclusion: Mastering Cycloalkane Nomenclature
Mastering IUPAC nomenclature for cycloalkanes is essential for success in organic chemistry. By understanding the basic principles, systematically following the naming steps, and practicing regularly, you can confidently select the correct IUPAC name for any cycloalkane, no matter the complexity. Remember to consider multiple substituents, numbering systems, alphabetical ordering, complex substituents, and stereochemistry to ensure a complete and accurate representation of the molecular structure. Consistent practice will lead to fluency in this critical aspect of organic chemistry.
Latest Posts
Latest Posts
-
Refer To Figure 4 17 At A Price Of
Mar 28, 2025
-
A Companys Ethical Code Of Conduct Is Not
Mar 28, 2025
-
One Makes An Attribution When One
Mar 28, 2025
-
Procedure 1 Tracing Blood Flow Patterns
Mar 28, 2025
-
A Payers Initial Processing Of A Claim Screens For
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Select The Correct Iupac Name For The Cycloalkane . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
