Rank The Structures In Order Of Decreasing Electrophilic Strength
Holbox
Mar 24, 2025 · 5 min read
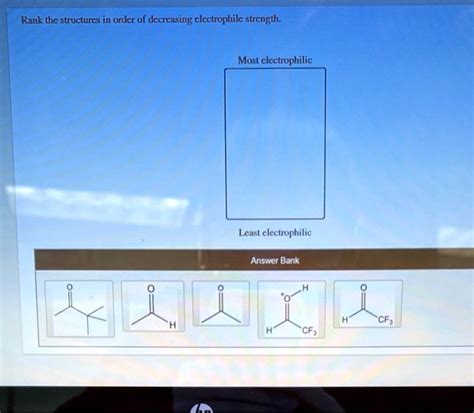
Table of Contents
Ranking Electrophilic Strength: A Comprehensive Guide
Electrophilicity, the tendency of an atom or molecule to attract electrons, is a fundamental concept in organic chemistry. Understanding the relative electrophilicity of different species is crucial for predicting reaction pathways and outcomes. This article provides a comprehensive ranking of various electrophilic structures in decreasing order of strength, explaining the underlying factors that influence their reactivity. We’ll explore various functional groups and structural features that impact electrophilicity. This guide aims to be a valuable resource for students and professionals alike in mastering this essential aspect of organic chemistry.
Factors Influencing Electrophilic Strength:
Before diving into the ranking, let's establish the key factors that determine the strength of an electrophile. These factors are intricately linked and often influence each other:
-
Formal Charge: A positive formal charge significantly enhances electrophilicity. The more positive the charge, the stronger the attraction for electrons. For example, a carbocation (R₃C⁺) is a much stronger electrophile than a neutral carbonyl compound (R₂C=O).
-
Electronegativity: Atoms with high electronegativity (like oxygen, nitrogen, and halogens) attract electrons more strongly. This can polarize a molecule, creating a partial positive charge on a specific atom, increasing its electrophilicity. This partial positive charge makes the molecule susceptible to nucleophilic attack.
-
Resonance: Delocalization of electrons through resonance can significantly affect electrophilicity. If the positive charge can be spread over multiple atoms, the electrophilicity is often reduced. Conversely, if the positive charge is localized on a single atom, the electrophilicity is increased.
-
Steric Hindrance: Bulky groups surrounding the electrophilic center can hinder the approach of nucleophiles, thereby reducing the effective electrophilicity. Steric hindrance can be a significant factor in determining reaction rates, even if the intrinsic electrophilicity is high.
-
Hybridization: The hybridization of the atom bearing the positive charge influences its electrophilicity. sp hybridized carbons are more electronegative than sp² or sp³ hybridized carbons, making them stronger electrophiles. This is because the sp hybridized carbon has more s-character, drawing electron density closer to the nucleus.
Ranking Electrophilic Structures:
The following ranking presents a general order of decreasing electrophilic strength. It's crucial to remember that the relative reactivity can be significantly influenced by the specific reaction conditions, solvents, and substituents present.
Tier 1: Extremely Strong Electrophiles:
-
Carbocations (R₃C⁺): These possess a full positive charge and are extremely reactive. Primary carbocations are less stable (and therefore more reactive) than secondary, which are less stable than tertiary carbocations. The instability drives their strong electrophilicity.
-
Acyl halides (RC(=O)X, where X = Cl, Br, I): The carbonyl carbon bears a significant partial positive charge due to the electronegativity of the halogen and the resonance effect within the carbonyl group. They readily undergo nucleophilic acyl substitution reactions.
-
Nitronium ion (NO₂⁺): This ion is a powerful electrophile involved in electrophilic aromatic substitution reactions, such as nitration. Its strong electrophilicity stems from the positive charge and the electron-withdrawing nature of the nitro group.
Tier 2: Strong Electrophiles:
-
Proton (H⁺): While seemingly simple, the proton is a highly reactive electrophile, particularly in acidic conditions. It readily adds to electron-rich species.
-
Alkyl halides (RX, where X = Cl, Br, I): Although less reactive than carbocations, the partial positive charge on the carbon atom bonded to the halogen makes them susceptible to nucleophilic attack, especially when the halogen is a good leaving group. Tertiary alkyl halides are generally more reactive than primary ones due to steric factors impacting the leaving group.
-
Sulfonium ions (R₃S⁺): These ions, similar to carbocations, have a positive charge on the sulfur atom, making them strong electrophiles.
-
Epoxides: The strained three-membered ring imparts a significant degree of reactivity to the epoxide. The carbon atoms bearing the oxygen are electrophilic, making them susceptible to nucleophilic attack, opening the ring.
Tier 3: Moderate Electrophiles:
-
Aldehydes and Ketones (RCHO and R₂C=O): The carbonyl carbon possesses a partial positive charge due to the electronegativity of oxygen. Their reactivity is moderate compared to carbocations or acyl halides, largely dependent on steric factors and the nature of the substituents.
-
Imines (R₂C=NR): The carbon atom in imines is partially positive due to the electron-withdrawing nature of the nitrogen atom. This allows them to react with nucleophiles.
-
Iminium ions (R₂C=NR₂⁺): The positive charge on the nitrogen further enhances the electrophilicity of the carbon atom compared to neutral imines.
Tier 4: Weak Electrophiles:
-
Alkenes and Alkynes: While generally considered nucleophiles, under specific conditions (e.g., in the presence of strong electrophiles or catalysts), the π electrons can act as weak nucleophiles, meaning the carbon atoms show weak electrophilic character.
-
Benzene and other Aromatic Compounds: Although aromatic compounds undergo electrophilic aromatic substitution, the π electron system is relatively stable, making the carbon atoms relatively weak electrophiles. The reaction requires strong electrophiles and specific reaction conditions.
-
Esters (RCOOR'): The carbonyl carbon possesses a partial positive charge, but the resonance stabilization of the carbonyl group reduces its electrophilicity compared to aldehydes and ketones.
Important Considerations:
-
Solvent Effects: The solvent can significantly influence the electrophilicity of a species by stabilizing or destabilizing charged intermediates. Polar solvents generally enhance the electrophilicity of positively charged species.
-
Nucleophile Strength: The strength of the nucleophile plays a crucial role in determining the reaction outcome. A strong nucleophile will react with weaker electrophiles, while a weaker nucleophile will only react with strong electrophiles.
-
Catalyst Influence: The presence of a catalyst can significantly enhance the electrophilicity of a species by activating it for nucleophilic attack. Lewis acids are commonly used to increase the electrophilicity of carbonyl compounds.
Conclusion:
Understanding the factors governing electrophilicity and having a general ranking of different functional groups is vital for successfully predicting the outcome of organic reactions. This guide provides a solid foundation for grasping this critical concept. However, it is crucial to remember that the relative reactivity of different electrophiles depends on many factors, and the ranking presented should be considered a general guideline rather than a rigid rule. Always consider the specific reaction conditions and the nature of the reacting species when evaluating the relative electrophilicity of different compounds. Further exploration of specific reactions and their mechanisms will reinforce this understanding. This provides a strong basis for advancing your comprehension of organic chemistry reactivity.
Latest Posts
Latest Posts
-
Match The Hormone Abbreviations With Their Function
Mar 26, 2025
-
Deserving Of Large Investment To Support Continued Growth
Mar 26, 2025
-
A Disadvantage Of Is That It
Mar 26, 2025
-
Which Of The Following Is Not A Manufacturing Cost Category
Mar 26, 2025
-
Pharmacotherapeutics For Advanced Practice A Practical Approach
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Rank The Structures In Order Of Decreasing Electrophilic Strength . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
