Rank The Leaving Groups Below From Worst To Best
Holbox
Mar 24, 2025 · 6 min read
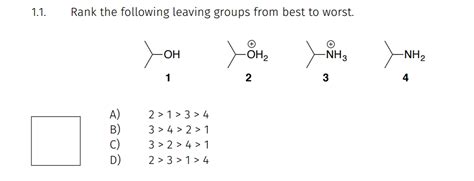
Table of Contents
- Rank The Leaving Groups Below From Worst To Best
- Table of Contents
- Ranking Leaving Groups: From Worst to Best
- Understanding Leaving Group Ability
- 1. Stability of the Conjugate Base:
- 2. Weak Basicity:
- 3. Solvent Effects:
- Ranking Leaving Groups: From Worst to Best
- Factors influencing leaving group ability: A deeper look
- Practical Implications and Examples
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Ranking Leaving Groups: From Worst to Best
Leaving groups are crucial in organic chemistry reactions, particularly substitution and elimination reactions. Understanding their relative abilities is fundamental to predicting reaction outcomes and designing synthetic strategies. This comprehensive guide will rank various leaving groups from worst to best, explaining the factors influencing their leaving group ability and providing examples for clarity.
Understanding Leaving Group Ability
A good leaving group is one that can stabilize the negative charge it acquires after leaving. This stability is directly related to several key factors:
1. Stability of the Conjugate Base:
The more stable the conjugate base (the species remaining after the leaving group departs), the better the leaving group. Stability is enhanced by factors like:
- Resonance: Groups capable of delocalizing the negative charge through resonance are excellent leaving groups. Examples include carboxylates (RCOO⁻) and sulfonates (like tosylate, mesylate, and triflate).
- Electronegativity: Highly electronegative atoms can better handle the negative charge. Halides (I⁻ > Br⁻ > Cl⁻ > F⁻) exemplify this, with iodide being the best halide leaving group due to its large size and diffuse charge.
- Size/Polarizability: Larger atoms can better distribute the negative charge, leading to increased stability. This is why iodide is a better leaving group than fluoride.
2. Weak Basicity:
Good leaving groups are weak bases. A strong base is reluctant to leave because it strongly wants to accept a proton. Conversely, a weak base is less likely to recapture a proton, making it a better leaving group. The weaker the conjugate base, the better the leaving group.
3. Solvent Effects:
The solvent can significantly impact leaving group ability. Protic solvents, which possess O-H or N-H bonds, can stabilize anions through hydrogen bonding, making them better leaving groups. Conversely, aprotic solvents may hinder the stabilization of anions, potentially affecting the leaving group's effectiveness.
Ranking Leaving Groups: From Worst to Best
Let's now rank a selection of common leaving groups, from worst to best. This ranking is a general guideline; specific reaction conditions might alter the relative order slightly.
Worst Leaving Groups:
-
Hydroxide (OH⁻): Hydroxide is a very strong base and a poor leaving group. It's highly reactive and prefers to remain bonded to the carbon atom. Reactions involving hydroxide as a leaving group often require strong conditions.
-
Alkoxide (RO⁻): Similar to hydroxide, alkoxides are strong bases and poor leaving groups. Their basicity hinders their departure. Their leaving group ability can be improved by protonation, converting them into a better leaving group, like water or an alcohol.
-
Amide (R₂N⁻): Amides are very strong bases and extremely poor leaving groups. Their high basicity makes them reluctant to depart.
-
Carbanions (R⁻): These are extremely strong bases and exceedingly poor leaving groups. They are highly reactive and strongly prefer to remain bonded to the carbon atom.
Intermediate Leaving Groups:
-
Water (H₂O): Water is a relatively weak base and a moderate leaving group. Its leaving group ability is enhanced in acidic conditions where protonation converts the hydroxyl group into a better leaving group (H₂O).
-
Alcohols (ROH): Alcohols are also weak bases and can act as moderate leaving groups, particularly after protonation. The protonation converts them into water, a much better leaving group.
-
Amines (RNH₂, R₂NH): Amines are relatively weak bases compared to hydroxide or alkoxides but are still weaker leaving groups than halides or sulfonates. Protonation to form an ammonium ion is beneficial for their departure.
Good Leaving Groups:
-
Fluoride (F⁻): While a halide, fluoride is a relatively poor leaving group due to its high electronegativity and small size which leads to high charge density and strong basicity. However, its leaving group ability can be significantly improved by using a strong Lewis acid.
-
Chloride (Cl⁻): Chloride is a much better leaving group than fluoride due to its larger size and better ability to distribute the negative charge. Its basicity is significantly lower than that of fluoride.
-
Bromide (Br⁻): Bromide is even better than chloride, exhibiting weaker basicity and better charge distribution.
-
Iodide (I⁻): Iodide is the best halide leaving group due to its large size, allowing for excellent charge distribution and leading to extremely weak basicity.
-
Sulfonates (Tosylate (OTs), Mesylate (OMs), Triflate (OTf)): Sulfonates are excellent leaving groups due to their resonance stabilization of the negative charge. Triflate (OTf) is generally considered the best of these due to the presence of three electronegative fluorine atoms. The electron-withdrawing properties of the sulfonate groups stabilize the negative charge effectively.
Factors influencing leaving group ability: A deeper look
The ranking above provides a general overview. However, the actual effectiveness of a leaving group can be influenced by various factors:
-
Steric hindrance: Bulky leaving groups might be hindered from departing due to steric congestion around the reaction site.
-
Solvent effects: Protic solvents generally stabilize anionic leaving groups better than aprotic solvents. The choice of solvent can drastically influence the rate of a reaction and the leaving group's efficiency.
-
Reaction conditions: The pH of the reaction medium is critical. Protonation can significantly enhance the leaving ability of some groups (e.g., converting alcohols and amines into better leaving groups).
-
Nature of the substrate: The electronic nature of the substrate (the molecule from which the leaving group departs) can impact the leaving group ability. Electron-withdrawing groups on the substrate can enhance the leaving group's departure by stabilizing the developing negative charge.
Practical Implications and Examples
Understanding leaving group ability is crucial for predicting the success and efficiency of many organic reactions. For example:
-
SN1 and SN2 reactions: In SN1 reactions, good leaving groups are essential because the reaction involves the formation of a carbocation intermediate. Good leaving groups stabilize this high-energy intermediate. SN2 reactions, which proceed through a concerted mechanism, are also influenced by leaving group ability—better leaving groups lead to faster reactions.
-
Elimination reactions (E1 and E2): Similar to substitution reactions, elimination reactions are also sensitive to leaving group ability. Good leaving groups favor the formation of alkenes.
-
Esterification and hydrolysis: The leaving group ability of the carboxylate group is critical in esterification reactions, where a better leaving group leads to a faster and more favorable equilibrium. Hydrolysis of esters also relies on the leaving group ability of the alkoxide group.
Conclusion
Leaving group ability is a multifaceted concept determined by a variety of factors. While a general ranking can provide guidance, the effectiveness of a leaving group in any specific reaction depends on the interplay of these factors, including the substrate, solvent, reaction mechanism, and specific conditions. A deep understanding of these nuances is crucial for effective synthetic planning and the prediction of reaction outcomes in organic chemistry. By considering these factors, chemists can design reactions that proceed efficiently and selectively, achieving the desired outcome.
Latest Posts
Latest Posts
-
Which Of The Following Statements About Platform Businesses Is True
Mar 26, 2025
-
At Some Instant And Location The Electric Field
Mar 26, 2025
-
Fundamentals Of Abnormal Psychology 10th Edition
Mar 26, 2025
-
Which Of The Following Statements About Equity Financing Is False
Mar 26, 2025
-
What Is Opsec Select All That Apply
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Rank The Leaving Groups Below From Worst To Best . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
