Rank The Following Ions In Order Of Increasing Basicity.
Holbox
Mar 21, 2025 · 5 min read
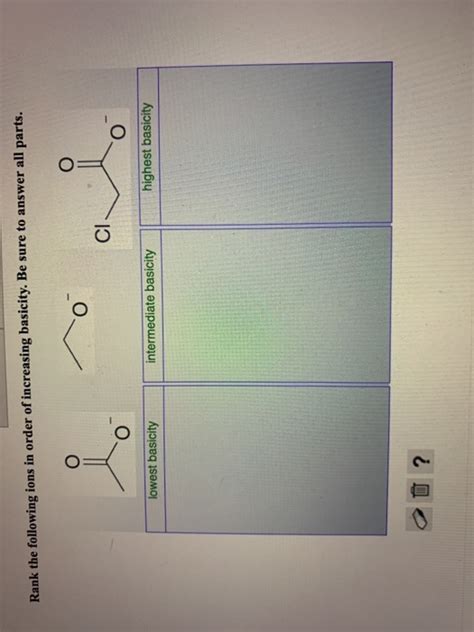
Table of Contents
Ranking Ions by Increasing Basicity: A Comprehensive Guide
Understanding basicity is crucial in chemistry, impacting various fields from industrial processes to biological systems. This article delves deep into the concept of basicity, explaining the factors that influence it, and providing a detailed, step-by-step approach to ranking ions based on their relative basicity. We'll explore several examples and address common misconceptions to build a strong foundational understanding.
What is Basicity?
Basicity refers to the ability of a substance to accept a proton (H⁺) or donate a pair of electrons. A stronger base readily accepts protons or donates electron pairs, while a weaker base does so less readily. This property is directly related to the ion's conjugate acid's strength; a stronger base has a weaker conjugate acid. The key factor to consider is the stability of the conjugate acid formed after protonation. A more stable conjugate acid indicates a weaker base, and vice versa.
Factors Affecting Basicity
Several factors contribute to an ion's basicity:
1. Charge Density:
Higher charge density correlates with stronger basicity. A more concentrated negative charge is more likely to attract a proton. For example, consider O²⁻ compared to OH⁻. O²⁻ possesses a higher charge density, making it a stronger base than OH⁻.
2. Electronegativity:
Electronegativity affects the ability of an atom to attract electrons. Less electronegative atoms hold onto electrons less tightly, making them better electron donors and thus stronger bases. For instance, nitrogen is less electronegative than oxygen, making NH₂⁻ a stronger base than OH⁻.
3. Resonance Effects:
Resonance delocalization of the negative charge can significantly impact basicity. If the negative charge is spread across multiple atoms, the charge density on any single atom decreases, reducing the ion's basicity. Conversely, ions without resonance stabilization are stronger bases because their negative charge is localized.
4. Inductive Effects:
Electron-donating groups increase basicity through inductive effects. These groups push electron density towards the negatively charged atom, increasing its ability to attract a proton. Conversely, electron-withdrawing groups decrease basicity.
5. Steric Hindrance:
Bulky groups surrounding the basic site can hinder protonation, reducing basicity. Steric hindrance makes it difficult for the proton to approach and bond with the basic site.
6. Hybridization:
The hybridization state of the atom carrying the negative charge influences basicity. Atoms with greater s-character are more electronegative and hold electrons more tightly, resulting in weaker basicity. For example, sp hybridized carbons are less basic than sp³ hybridized carbons.
Ranking Ions: A Step-by-Step Approach
To rank a series of ions by increasing basicity, systematically analyze each ion based on the above factors. Let's consider a hypothetical example:
Rank the following ions in order of increasing basicity: CH₃⁻, NH₂⁻, OH⁻, F⁻
Step 1: Analyze Charge Density:
All ions carry a -1 charge, so this factor doesn't differentiate basicity in this case.
Step 2: Analyze Electronegativity:
The electronegativity trend is F > O > N > C. Fluorine is the most electronegative, meaning it holds onto its negative charge more tightly, making it the weakest base. Carbon is the least electronegative, making CH₃⁻ the strongest base.
Step 3: Consider Resonance and Inductive Effects:
None of these ions exhibit significant resonance or inductive effects that would significantly alter their basicity.
Step 4: Analyze Steric Hindrance:
Steric hindrance is negligible in this example.
Step 5: Analyze Hybridization:
All atoms are sp³ hybridized, so this factor doesn't differentiate basicity.
Step 6: Combine Factors and Rank:
Based on electronegativity, the order of increasing basicity is: F⁻ < OH⁻ < NH₂⁻ < CH₃⁻
Therefore, the final ranking is: F⁻ < OH⁻ < NH₂⁻ < CH₃⁻
Illustrative Examples and Deeper Analysis
Let's consider some more complex examples, incorporating more of the factors discussed earlier:
Example 1: Ranking Acetate (CH₃COO⁻), Trifluoroacetate (CF₃COO⁻), and Methoxide (CH₃O⁻)
-
Acetate (CH₃COO⁻): The negative charge is delocalized across two oxygen atoms through resonance, reducing its basicity. The methyl group exhibits a weak electron-donating inductive effect, slightly increasing basicity.
-
Trifluoroacetate (CF₃COO⁻): The trifluoromethyl group (CF₃) is strongly electron-withdrawing due to the high electronegativity of fluorine. This effect significantly reduces the electron density on the carboxylate group, making it a much weaker base than acetate. Resonance stabilization further weakens its basicity.
-
Methoxide (CH₃O⁻): The negative charge is localized on the oxygen atom. The methyl group exhibits a weak electron-donating effect, slightly increasing basicity.
Ranking: CF₃COO⁻ < CH₃COO⁻ < CH₃O⁻
Example 2: Ranking Amide (RCONH⁻) and Carbanion (RCH₂⁻)
-
Amide (RCONH⁻): The negative charge is delocalized through resonance with the carbonyl oxygen, substantially decreasing basicity.
-
Carbanion (RCH₂⁻): The negative charge is localized on the carbon atom. The basicity depends largely on the nature of the R group – electron-donating groups would enhance basicity, while electron-withdrawing groups would reduce it.
Ranking (Assuming a simple alkyl R group): RCONH⁻ < RCH₂⁻
Example 3: Ranking different substituted anilines
The basicity of anilines is heavily influenced by substituents on the benzene ring. Electron-donating groups increase basicity, while electron-withdrawing groups decrease it.
For example, p-methylaniline (with an electron-donating methyl group) is more basic than aniline, while p-nitroaniline (with an electron-withdrawing nitro group) is significantly less basic than aniline.
Advanced Considerations
In more complex scenarios, computational chemistry methods, such as density functional theory (DFT) calculations, can provide accurate predictions of pKa values, which are directly related to basicity. These methods can account for subtle electronic and steric effects that are challenging to predict qualitatively.
Conclusion
Ranking ions by increasing basicity requires a systematic approach that considers several interrelated factors: charge density, electronegativity, resonance, inductive effects, steric hindrance, and hybridization. By carefully analyzing these factors for each ion, one can accurately predict the order of increasing basicity. Understanding these principles is not only important for academic pursuits but also for practical applications in various fields, including organic synthesis, biochemistry, and materials science. The examples and in-depth explanations provided here aim to equip you with the knowledge and tools necessary to confidently approach these types of problems. Remember to always consider all contributing factors and apply your understanding strategically for accurate ranking.
Latest Posts
Latest Posts
-
Select The Best Conditions For The Reactions
Mar 21, 2025
-
The Fluid In The Anterior Cavity Is Known As
Mar 21, 2025
-
Two Main Inventory Accounting Systems Are The
Mar 21, 2025
-
Which Of The Following Are Not Secreted By Platelets
Mar 21, 2025
-
Name The Vertebral Projection Oriented In A Sagittal Plane
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Rank The Following Ions In Order Of Increasing Basicity. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
