Question Violet What Is The Multiplicity Of The Methyl Peak
Holbox
Mar 13, 2025 · 6 min read
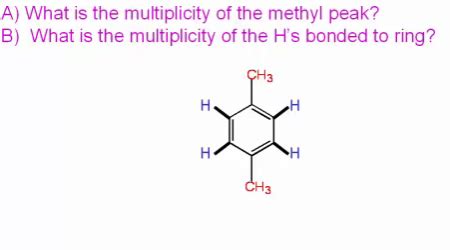
Table of Contents
Question Violet: What is the Multiplicity of the Methyl Peak? Understanding NMR Spectroscopy
Nuclear Magnetic Resonance (NMR) spectroscopy is a powerful analytical technique used extensively in chemistry and related fields to determine the structure of organic molecules. A key aspect of interpreting NMR spectra is understanding the multiplicity of signals, particularly the multiplicity of methyl peaks. This article delves deep into the concept of methyl peak multiplicity in NMR spectroscopy, explaining the underlying principles and providing practical examples to enhance your understanding.
Understanding NMR Spectroscopy Basics
Before we dive into the multiplicity of methyl peaks, let's briefly revisit the fundamental principles of NMR spectroscopy. NMR spectroscopy exploits the magnetic properties of certain atomic nuclei. Specifically, it focuses on nuclei with a non-zero spin quantum number, such as ¹H (proton) and ¹³C (carbon-13).
When placed in a strong external magnetic field, these nuclei can absorb radiofrequency (RF) radiation at specific frequencies, which depend on their chemical environment. This absorption of energy causes the nuclei to transition between different spin states. The resulting spectrum provides valuable information about the number, type, and connectivity of atoms in a molecule.
The chemical shift in an NMR spectrum indicates the resonance frequency of a nucleus relative to a standard reference compound (like tetramethylsilane, TMS). Different chemical environments cause nuclei to resonate at slightly different frequencies, leading to distinct peaks in the spectrum.
The Concept of Spin-Spin Coupling
One of the most crucial aspects of interpreting NMR spectra is understanding spin-spin coupling. This phenomenon arises from the interaction between the magnetic moments of neighboring nuclei. The magnetic field experienced by a given nucleus is influenced by the spin states of its neighboring nuclei. This interaction leads to a splitting of the NMR signal into multiple peaks, giving rise to the multiplicity of the signal.
The multiplicity of a peak is determined by the n+1 rule, where 'n' is the number of equivalent neighboring protons. This rule applies to first-order coupling, which is the most common type encountered in simple organic molecules.
Example: A proton with three equivalent neighboring protons will exhibit a quartet (n+1 = 3+1 = 4 peaks).
Methyl Peak Multiplicity: A Detailed Explanation
Methyl groups (–CH₃) are common functional groups in organic molecules. Understanding the multiplicity of the methyl peak in an NMR spectrum is crucial for structure elucidation. The multiplicity of a methyl peak depends entirely on the number of neighboring protons that are chemically distinct and not equivalent to those in the methyl group. Remember, the methyl protons themselves are considered equivalent.
Let's explore different scenarios to illustrate this point:
1. Methyl Group with No Neighboring Protons
If a methyl group has no neighboring protons (i.e., no protons on adjacent carbon atoms), the methyl peak will be a singlet. This is because there are no neighboring protons to interact with, and the n+1 rule gives 0+1=1 peak.
Example: In the NMR spectrum of (CH₃)₄Si (tetramethylsilane), the methyl peak is a singlet.
2. Methyl Group with One Neighboring Proton
If a methyl group has one neighboring proton, the methyl peak will be a doublet (n+1 = 1+1 = 2 peaks). The splitting pattern reflects the two possible spin states (+1/2 and -1/2) of the neighboring proton.
Example: Consider a molecule with a –CH₂–CH₃ fragment. The methyl peak will be a doublet due to coupling with the single adjacent proton on the CH₂ group.
3. Methyl Group with Two Neighboring Equivalent Protons
If a methyl group has two equivalent neighboring protons, the methyl peak will appear as a triplet (n+1 = 2+1 = 3 peaks). The three peaks reflect the three possible spin states of the two equivalent neighboring protons: (+1/2, +1/2), (+1/2, -1/2), and (-1/2, -1/2).
Example: Consider a molecule with a –CH₂–CH₂–CH₃ fragment. The methyl protons will couple with the two equivalent protons on the adjacent methylene group, producing a triplet.
4. Methyl Group with Three Neighboring Equivalent Protons
If a methyl group has three equivalent neighboring protons, the methyl peak will appear as a quartet (n+1 = 3+1 = 4 peaks).
Example: A molecule with a -CH₂-CH₂-CH₃ fragment illustrates this. However, it is less common to directly couple to three equivalent protons unless involved in a specific structural arrangement. Often, complex splitting patterns emerge from more intricate interactions, potentially obscuring this simple quartet pattern.
5. Methyl Groups with Multiple Sets of Neighboring Protons
In more complex molecules, the methyl group might have multiple sets of neighboring protons, leading to more complex splitting patterns. The overall multiplicity would then be the product of the individual splitting patterns, subject to factors like coupling constants and peak overlap. These situations frequently necessitate more advanced NMR analysis techniques to fully decipher the spectrum.
Factors Affecting Methyl Peak Multiplicity
Several factors can influence the observed multiplicity of a methyl peak:
-
Coupling Constants: The magnitude of the coupling constant (J), which represents the strength of the interaction between nuclei, determines the spacing between the peaks in a multiplet. Larger coupling constants result in greater peak separation.
-
Peak Overlap: If the chemical shifts of different protons are very close, their signals might overlap, making it difficult to discern the true multiplicity.
-
Higher-Order Coupling: In some cases, coupling to more than one type of neighboring proton or long-range coupling (coupling over more than three bonds) can lead to more complex and less easily predictable splitting patterns.
-
Exchange Processes: In molecules involving rapid exchange processes (such as proton exchange in acidic or basic conditions), the spin-spin coupling can be averaged out, leading to simplified peak patterns, sometimes even eliminating the splitting entirely.
Practical Applications and Examples
The understanding of methyl peak multiplicity is critical in several applications:
-
Structure Elucidation: The multiplicity of the methyl peak provides valuable information about the neighboring groups and the connectivity of atoms in a molecule.
-
Quantitative Analysis: The integration of the methyl peak can give information about the relative abundance of different molecular species in a mixture.
-
Reaction Monitoring: NMR spectroscopy, specifically monitoring the multiplicity and integration of the methyl peak, can track the progress of chemical reactions.
Advanced Concepts and Further Considerations
For more complex molecules and scenarios, more sophisticated NMR techniques are required to fully resolve and interpret the spectra. These techniques can include:
-
Two-Dimensional NMR Spectroscopy: Techniques like COSY (Correlation Spectroscopy) and HSQC (Heteronuclear Single Quantum Coherence) can reveal the connectivity of protons and provide a clearer understanding of complex spin systems.
-
Advanced Pulse Sequences: Various pulse sequences can be employed to selectively suppress or enhance certain signals, simplifying complex spectra.
Conclusion
Understanding the multiplicity of the methyl peak is essential for interpreting NMR spectra accurately. This knowledge allows chemists and researchers to determine the structure of molecules, monitor reactions, and perform quantitative analysis. While the basic n+1 rule serves as a helpful guide, a deep understanding of the factors affecting multiplicity and more advanced NMR techniques is crucial for tackling more complex molecular structures and spectral analyses. Remember to always consider coupling constants, peak overlaps, and potential for higher-order effects when deciphering your spectral data. This comprehensive understanding of methyl peak multiplicity contributes significantly to the overall interpretation of NMR spectroscopy data and expands your toolkit for advanced structural analysis.
Latest Posts
Latest Posts
-
A Person Drops A Vertically Oriented Cylindrical Steel Bar
Mar 13, 2025
-
Provide The Correct Iupac Name For The Compound Shown Here
Mar 13, 2025
-
An Example Of An Individual Financial Coi Is
Mar 13, 2025
-
Research On Bias Throughout The Child Welfare System Shows
Mar 13, 2025
-
An Example Of An Institutional Coi Is
Mar 13, 2025
Related Post
Thank you for visiting our website which covers about Question Violet What Is The Multiplicity Of The Methyl Peak . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
