Provide The Correct Iupac Name For The Compound Shown Here
Holbox
Mar 24, 2025 · 5 min read
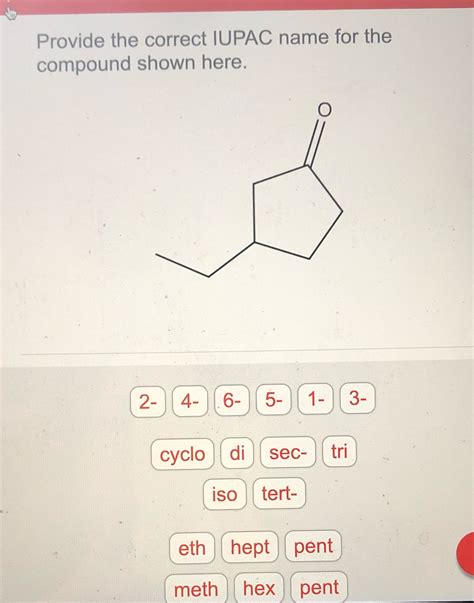
Table of Contents
- Provide The Correct Iupac Name For The Compound Shown Here
- Table of Contents
- Providing the Correct IUPAC Name for Organic Compounds: A Comprehensive Guide
- Understanding the Fundamentals of IUPAC Nomenclature
- 1. Identifying the Parent Chain: The Foundation of Nomenclature
- 2. Identifying Functional Groups: Defining the Compound Type
- 3. Locating and Naming Substituents: Adding Detail to the Name
- Step-by-Step Guide to Assigning IUPAC Names
- Examples of IUPAC Nomenclature
- Advanced Considerations in IUPAC Nomenclature
- Conclusion: Mastering the Art of IUPAC Nomenclature
- Latest Posts
- Related Post
Providing the Correct IUPAC Name for Organic Compounds: A Comprehensive Guide
Determining the correct IUPAC name for an organic compound can seem daunting, especially when faced with complex structures. However, with a systematic approach and a solid understanding of IUPAC nomenclature rules, this task becomes significantly more manageable. This article provides a comprehensive guide to naming organic compounds, focusing on accuracy and clarity to ensure you can confidently assign the correct IUPAC name to any given structure. We will delve into the fundamental principles, essential steps, and practical examples to solidify your understanding.
Understanding the Fundamentals of IUPAC Nomenclature
The International Union of Pure and Applied Chemistry (IUPAC) developed a standardized system for naming organic compounds to ensure global consistency and avoid ambiguity. This system, known as IUPAC nomenclature, follows a set of rules based on the structure of the molecule. The key principles involve identifying the parent chain, functional groups, substituents, and their positions within the molecule.
1. Identifying the Parent Chain: The Foundation of Nomenclature
The parent chain forms the backbone of the IUPAC name. This is typically the longest continuous carbon chain in the molecule. In cases of ties, prioritize the chain with the most substituents. Consider the following example:
Example: A molecule with a six-carbon chain and a five-carbon chain with more substituents would use the five-carbon chain as the parent chain despite its shorter length.
2. Identifying Functional Groups: Defining the Compound Type
Functional groups are specific groups of atoms within a molecule that determine its chemical properties and reactivity. These groups have specific prefixes or suffixes that are incorporated into the IUPAC name to indicate their presence. Common functional groups include:
- Hydrocarbons: These contain only carbon and hydrogen atoms. They can be alkanes (single bonds), alkenes (double bonds), or alkynes (triple bonds).
- Alcohols (-OH): The suffix "-ol" is added to the parent alkane name.
- Ketones (C=O): The suffix "-one" is added, and the position of the carbonyl group is indicated by a number.
- Aldehydes (CHO): The suffix "-al" is added.
- Carboxylic acids (-COOH): The suffix "-oic acid" is added.
- Amines (-NH2): The prefix "amino-" is used.
- Ethers (-O-): The prefix "oxy-" is used, indicating the position of the oxygen atom connecting the two alkyl groups.
3. Locating and Naming Substituents: Adding Detail to the Name
Substituents are atoms or groups of atoms attached to the parent chain that are not part of the primary functional group. Their positions are indicated by numbers, starting from the end of the chain closest to the highest priority functional group. If multiple substituents are present, they are listed alphabetically, ignoring prefixes like di-, tri-, etc., unless they are part of a complex substituent name (e.g., isopropyl).
Step-by-Step Guide to Assigning IUPAC Names
Let's walk through a systematic process for assigning IUPAC names:
Step 1: Identify the Parent Chain
Determine the longest continuous carbon chain in the molecule.
Step 2: Identify the Principal Functional Group
Determine the functional group with the highest priority according to IUPAC rules. This group will dictate the suffix of the name. The order of priority generally follows carboxylic acids > aldehydes > ketones > alcohols > amines > ethers > alkenes > alkynes > alkanes.
Step 3: Number the Carbon Atoms
Number the carbon atoms in the parent chain, starting from the end closest to the highest-priority functional group or the substituent with alphabetical priority.
Step 4: Identify and Name Substituents
Identify all substituents attached to the parent chain. Assign numbers to indicate their position on the chain. Name each substituent according to its structure.
Step 5: Arrange Substituents Alphabetically
List the substituents in alphabetical order, ignoring prefixes like di-, tri-, etc. (except when part of a complex substituent name). Use hyphens to separate numbers and names. Use commas to separate numbers.
Step 6: Combine the Information
Combine the information gathered to create the IUPAC name. The general format is:
[Prefixes (substituents) + locants (numbers)] + [parent chain name] + [suffix (functional group)]
Examples of IUPAC Nomenclature
Let's illustrate this process with some examples:
Example 1:
Consider a molecule with a four-carbon chain, a hydroxyl group (-OH) on the second carbon, and a methyl group (-CH3) on the third carbon.
- Parent chain: Butane (4 carbons)
- Principal functional group: Alcohol (-OH)
- Numbering: Numbering starts from the end closest to the -OH group.
- Substituents: Methyl group on carbon 3.
- Alphabetical arrangement: Methyl.
- Combined name: 3-Methylbutan-2-ol
Example 2: A more complex molecule with multiple substituents and a different functional group.
(Imagine a molecule here with a longer chain, multiple substituents like ethyl, propyl, chloro etc, and a ketone functional group. You would then systematically follow the steps above to determine the IUPAC name. The complexity will increase, requiring careful attention to numbering and alphabetical ordering. This example is best illustrated with a diagram, which is not feasible in this markdown format.)
Example 3: A molecule containing a ring structure.
(Similarly, a complex molecule with a ring structure would require a diagram for accurate representation and would be named using similar principles, but the parent chain would be identified as the ring structure. Specific ring nomenclature rules apply. Again, a diagram is essential for clear explanation.)
Advanced Considerations in IUPAC Nomenclature
Several more advanced concepts exist within IUPAC nomenclature that we haven't covered in detail:
- Stereochemistry: This involves specifying the spatial arrangement of atoms in a molecule (e.g., cis/trans isomers, R/S configurations).
- Complex Substituents: Naming complex substituents requires a hierarchical approach, treating the substituent itself as a parent chain.
- Bridged Ring Systems: Specific rules govern the naming of molecules with multiple fused or bridged rings.
Conclusion: Mastering the Art of IUPAC Nomenclature
Mastering IUPAC nomenclature is crucial for any student or professional working with organic chemistry. This guide provides a foundational understanding of the principles and steps involved in accurately naming organic compounds. Remember that practice is key. The more examples you work through, the more confident and proficient you'll become in applying these rules and naming even the most complex molecules correctly. Remember to consult an advanced organic chemistry textbook or online resources for additional support and practice problems. The systematic approach outlined here, along with diligent practice, will enable you to confidently assign the correct IUPAC name to a variety of organic compounds.
Latest Posts
Related Post
Thank you for visiting our website which covers about Provide The Correct Iupac Name For The Compound Shown Here . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.