Question Jon Draw The Major Organic Product
Holbox
Mar 13, 2025 · 6 min read
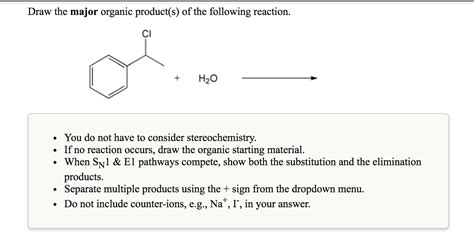
Table of Contents
Question: Jon Draws the Major Organic Product – A Deep Dive into Organic Chemistry Reaction Mechanisms
Organic chemistry can be daunting, especially when faced with complex reaction mechanisms and predicting the major organic product. This article delves into the crucial process of predicting the major product in organic reactions, focusing on understanding reaction mechanisms and employing common strategies to accurately determine the outcome. We'll explore several reaction types, including substitution, addition, and elimination reactions, and address factors influencing product distribution. Let's tackle Jon's challenge and learn to master the art of predicting major organic products.
Understanding Reaction Mechanisms: The Key to Predicting Products
Before diving into specific reactions, it’s crucial to understand the underlying mechanism. A reaction mechanism describes the step-by-step process of bond breaking and bond formation during a chemical transformation. Knowing the mechanism helps us predict the structure of the intermediate(s) and, ultimately, the final product(s).
Key Concepts in Reaction Mechanisms:
- Nucleophiles: Electron-rich species that donate electrons to electron-deficient atoms (electrophiles). Examples include hydroxide ions (OH⁻), alcohols (ROH), and amines (RNH₂).
- Electrophiles: Electron-deficient species that accept electrons from nucleophiles. Examples include carbocations (R₃C⁺), carbonyl carbons (C=O), and alkyl halides (RX).
- Leaving Groups: Atoms or groups that depart from a molecule, taking an electron pair with them. Common leaving groups include halides (Cl⁻, Br⁻, I⁻), water (H₂O), and tosylate (OTs).
- Carbocation Stability: Carbocations (positively charged carbon atoms) are key intermediates in many reactions. Their stability influences the reaction pathway and product formation. Tertiary carbocations are the most stable, followed by secondary, then primary, with methyl carbocations being the least stable. Resonance stabilization further enhances carbocation stability.
- Transition States: High-energy, short-lived species representing the peak of the energy barrier in a reaction pathway. They are not isolable but crucial for understanding reaction rates and selectivity.
Common Reaction Types and Predicting Major Products
Let's examine several common reaction types and strategies for identifying the major organic product.
1. SN1 and SN2 Reactions (Nucleophilic Substitution)
These reactions involve the substitution of a leaving group by a nucleophile. The key difference lies in the mechanism:
-
SN1 (Substitution Nucleophilic Unimolecular): This reaction proceeds in two steps. The first step involves the departure of the leaving group, forming a carbocation intermediate. The second step involves the attack of the nucleophile on the carbocation. SN1 reactions favor tertiary substrates due to the greater stability of the resulting tertiary carbocation. Racemization (formation of both enantiomers) is often observed because the nucleophile can attack the planar carbocation from either side.
-
SN2 (Substitution Nucleophilic Bimolecular): This reaction proceeds in a single step, with the nucleophile attacking the substrate from the backside while the leaving group departs. SN2 reactions favor primary substrates because steric hindrance hinders backside attack in secondary and tertiary substrates. SN2 reactions result in inversion of configuration (Walden inversion).
Predicting the major product in SN1 and SN2 reactions requires considering:
- Substrate structure: Primary, secondary, or tertiary alkyl halide.
- Nucleophile strength and sterics: Strong nucleophiles favor SN2, while weak nucleophiles favor SN1. Bulky nucleophiles hinder SN2 reactions.
- Solvent: Polar protic solvents favor SN1, while polar aprotic solvents favor SN2.
2. E1 and E2 Reactions (Elimination Reactions)
Elimination reactions involve the removal of a leaving group and a proton from adjacent carbon atoms, forming a double bond (alkene).
-
E1 (Elimination Unimolecular): This two-step mechanism involves the formation of a carbocation intermediate, followed by the abstraction of a proton by a base. E1 reactions favor tertiary substrates due to the greater stability of the resulting carbocation. The more substituted alkene is typically the major product (Zaitsev's rule).
-
E2 (Elimination Bimolecular): This concerted, one-step mechanism involves the simultaneous removal of the proton and leaving group by a base. The stereochemistry is crucial; the proton and leaving group must be anti-periplanar (on opposite sides of the molecule). E2 reactions can occur with primary, secondary, and tertiary substrates. Zaitsev's rule generally predicts the major product.
Predicting the major product in E1 and E2 reactions requires considering:
- Substrate structure: Primary, secondary, or tertiary alkyl halide.
- Base strength and sterics: Strong, bulky bases favor Hofmann elimination (less substituted alkene), while strong, less bulky bases favor Zaitsev elimination (more substituted alkene).
- Temperature: Higher temperatures favor E1 and E2 reactions.
3. Addition Reactions
Addition reactions involve the addition of atoms or groups to a double or triple bond. The most common type is electrophilic addition to alkenes.
- Electrophilic Addition to Alkenes: This reaction proceeds through a two-step mechanism. The electrophile attacks the double bond, forming a carbocation intermediate. A nucleophile then attacks the carbocation, resulting in the addition of the electrophile and nucleophile across the double bond. Markovnikov's rule states that the electrophile adds to the carbon atom with the greater number of hydrogen atoms. Markovnikov's rule is violated in cases involving radical additions.
Predicting the major product in addition reactions requires considering:
- Substrate structure: The structure of the alkene influences the stability of the carbocation intermediate.
- Reagent: The nature of the electrophile and nucleophile influences the regioselectivity (where the atoms add).
4. Grignard Reactions
Grignard reactions involve the reaction of an organomagnesium halide (Grignard reagent) with a carbonyl compound (aldehyde or ketone). The Grignard reagent acts as a nucleophile, attacking the carbonyl carbon. The resulting alkoxide is then protonated to yield an alcohol.
Predicting the major product in Grignard reactions involves:
- Identifying the carbonyl compound: Aldehydes yield secondary alcohols, while ketones yield tertiary alcohols.
- Considering steric hindrance: Bulky Grignard reagents may react slower or give different product distribution.
Factors Influencing Product Distribution
Several factors beyond the basic reaction mechanism can influence the product distribution:
- Steric effects: Bulky groups can hinder reactions and influence the regioselectivity and stereoselectivity of the reaction.
- Electronic effects: Electron-donating or withdrawing groups on the substrate can influence the reactivity and selectivity.
- Temperature and solvent: These factors can affect the reaction rate and favor different pathways.
- Catalyst: Catalysts can accelerate reactions and alter the product distribution by modifying the reaction pathway or stabilizing intermediates.
Putting it all Together: A Systematic Approach
To accurately predict the major organic product, follow these steps:
- Identify the functional groups: Determine the reacting functional groups in the starting material and reagents.
- Identify the reaction type: Classify the reaction as substitution, addition, or elimination.
- Determine the reaction mechanism: Analyze the mechanism (SN1, SN2, E1, E2, etc.) based on the structure of the substrate, the reagents, and the reaction conditions.
- Predict the intermediate(s): Identify the key intermediates formed during the reaction.
- Predict the product(s): Based on the intermediate(s) and reaction mechanism, predict the structure of the product(s). Consider regioselectivity (which atom the reagent adds to) and stereoselectivity (which stereoisomer is formed).
- Consider the factors influencing product distribution: Take into account steric and electronic effects, temperature, solvent, and catalysts.
- Determine the major product: Based on the relative rates of formation and stability of different products, identify the major product.
By following this systematic approach, Jon (and you!) can confidently predict the major organic products in a wide variety of reactions. Remember, practice is key. Work through many examples to solidify your understanding and improve your ability to anticipate reaction outcomes. Mastering organic chemistry is a journey, not a sprint, but with consistent effort and a thorough understanding of reaction mechanisms, you can overcome the challenges and become proficient in predicting reaction products.
Latest Posts
Latest Posts
-
Linear Modeling Of Nyc Mta Transit Fares
Mar 13, 2025
-
Which Is Not A Temporary Account
Mar 13, 2025
-
Selection Of Incident Commanders Is Done By The
Mar 13, 2025
-
Drag The Labels To The Appropriate Location In The Figure
Mar 13, 2025
-
Which Formula Name Pair Is Incorrect
Mar 13, 2025
Related Post
Thank you for visiting our website which covers about Question Jon Draw The Major Organic Product . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
