Question Cherry Select The Carbanion That Is The Weakest Base
Holbox
Mar 14, 2025 · 5 min read
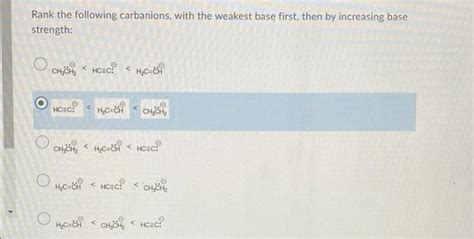
Table of Contents
Question: Cherry-Select the Carbanion that is the Weakest Base
Understanding the relative basicity of carbanions is crucial in organic chemistry. Carbanions, species with a negatively charged carbon atom, are strong nucleophiles and bases. Their reactivity is heavily influenced by factors such as resonance stabilization, inductive effects, and hybridization. This article delves into the principles governing carbanion basicity and guides you through selecting the weakest base from a given set of options. We'll explore various examples to solidify your understanding.
Understanding Carbanion Basicity
The strength of a base is directly related to its ability to accept a proton (H⁺). A weaker base is less likely to abstract a proton compared to a stronger base. Several factors influence carbanion basicity:
1. Resonance Stabilization:
Carbanions possessing resonance structures are significantly more stable than those without. Delocalization of the negative charge across multiple atoms reduces electron density on the carbanionic carbon, decreasing its basicity. The more resonance structures available, the weaker the base.
Example: Compare the basicity of a simple alkyl carbanion (e.g., CH₃⁻) with that of a benzyl carbanion (C₆H₅CH₂⁻). The benzyl carbanion is significantly less basic due to resonance stabilization. The negative charge can be delocalized into the aromatic ring, effectively reducing its nucleophilicity and basicity.
2. Inductive Effects:
Electron-withdrawing groups (EWGs) attached to the carbanionic carbon stabilize the negative charge through inductive effects, reducing basicity. Conversely, electron-donating groups (EDGs) destabilize the negative charge, increasing basicity.
Example: Consider the following carbanions: CH₃CH₂⁻, CH₃CHF⁻, CH₃CCl₂⁻. The electronegative fluorine and chlorine atoms withdraw electron density from the carbanionic carbon. Therefore, CH₃CCl₂⁻ is the weakest base, followed by CH₃CHF⁻, with CH₃CH₂⁻ being the strongest base. The more electronegative the halogen, the stronger the inductive effect and the weaker the base.
3. Hybridization:
The hybridization of the carbanionic carbon also affects its basicity. sp hybridized carbons are more electronegative than sp² or sp³ hybridized carbons. Consequently, carbanions with sp hybridization are less basic than those with sp² or sp³ hybridization. This is because the higher s-character in the sp orbital pulls the electron density closer to the nucleus.
Example: Compare the basicity of an acetylide ion (sp hybridized) with a simple alkyl carbanion (sp³ hybridized). The acetylide ion (e.g., HC≡C⁻) is a weaker base than the alkyl carbanion (e.g., CH₃⁻) because the sp hybridized carbon holds the negative charge more tightly.
4. Steric Hindrance:
Bulky substituents around the carbanionic carbon can hinder the approach of a proton, making it a weaker base. Steric hindrance reduces the accessibility of the negative charge to a proton.
Example: A tertiary carbanion (e.g., (CH₃)₃C⁻) is generally a weaker base than a primary carbanion (e.g., CH₃CH₂⁻) due to increased steric hindrance around the carbanionic carbon.
Selecting the Weakest Base: A Step-by-Step Approach
To identify the weakest base from a group of carbanions, systematically evaluate the factors influencing basicity:
-
Identify Resonance Stabilization: Look for carbanions with resonance structures. Those with extensive resonance are weaker bases.
-
Assess Inductive Effects: Examine the substituents attached to the carbanionic carbon. Electron-withdrawing groups (EWGs) weaken the base, while electron-donating groups (EDGs) strengthen it. Consider the electronegativity and the number of EWGs.
-
Determine Hybridization: Note the hybridization of the carbanionic carbon. sp hybridized carbons lead to weaker bases compared to sp² and sp³ hybridized carbons.
-
Consider Steric Effects: Evaluate the steric bulk around the carbanionic carbon. Increased steric hindrance reduces basicity.
-
Combine Factors: Once you've considered each factor, combine the effects to determine the overall basicity. The carbanion with the greatest degree of stabilization (through resonance, inductive effects, or hybridization) and/or significant steric hindrance will be the weakest base.
Practice Examples
Let's apply these principles to some examples. Identify the weakest base in each set:
Example 1:
- CH₃⁻
- CH₂=CH⁻
- HC≡C⁻
- C₆H₅CH₂⁻
Solution:
- CH₃⁻: sp³ hybridized, no resonance, no significant inductive effects.
- CH₂=CH⁻: sp² hybridized, some resonance, no significant inductive effects.
- HC≡C⁻: sp hybridized, no resonance, no significant inductive effects.
- C₆H₅CH₂⁻: sp³ hybridized, significant resonance stabilization.
Weakest Base: C₆H₅CH₂⁻ (due to strong resonance stabilization).
Example 2:
- CH₃CH₂⁻
- CH₃CHCl⁻
- CH₃CHF⁻
- (CH₃)₃C⁻
Solution:
- CH₃CH₂⁻: sp³ hybridized, no resonance or significant inductive effects.
- CH₃CHCl⁻: sp³ hybridized, inductive effect from Cl.
- CH₃CHF⁻: sp³ hybridized, inductive effect from F (stronger than Cl).
- (CH₃)₃C⁻: sp³ hybridized, steric hindrance.
Weakest Base: CH₃CHF⁻ (stronger inductive effect from F). While (CH₃)₃C⁻ has steric hindrance, the inductive effect of fluorine is a more dominant factor in this case.
Example 3:
- CH₃COO⁻
- CH₃CH₂O⁻
- CH₃⁻
- PhO⁻ (Phenoxide ion)
Solution:
- CH₃COO⁻: sp² hybridized, strong resonance stabilization (carboxylate ion).
- CH₃CH₂O⁻: sp³ hybridized, weaker base compared to alkyl carbanion.
- CH₃⁻: sp³ hybridized, no resonance or significant inductive effects.
- PhO⁻: sp² hybridized, resonance stabilization (phenoxide ion).
Weakest Base: CH₃COO⁻ (strong resonance stabilization).
Conclusion
Determining the weakest base among carbanions requires a careful consideration of resonance stabilization, inductive effects, hybridization, and steric hindrance. By systematically analyzing these factors, you can accurately predict the relative basicity of different carbanions. This understanding is fundamental to comprehending organic reaction mechanisms and designing synthetic strategies. Remember to practice with various examples to solidify your understanding and develop your problem-solving skills in organic chemistry. The more you practice, the more intuitive this process will become. Mastering this concept is a crucial step toward achieving proficiency in organic chemistry.
Latest Posts
Latest Posts
-
All Of The Following Are Disadvantages Of A Corporation Except
Mar 14, 2025
-
Planning Value If Range Is Givn
Mar 14, 2025
-
All Of The Following Are True Except
Mar 14, 2025
-
Manufacturing Costs Include Direct Materials Direct Labor And
Mar 14, 2025
-
Health Screenings Are Important Measures In
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about Question Cherry Select The Carbanion That Is The Weakest Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
