Provide The Formula For Each Compound.
Holbox
Mar 15, 2025 · 6 min read
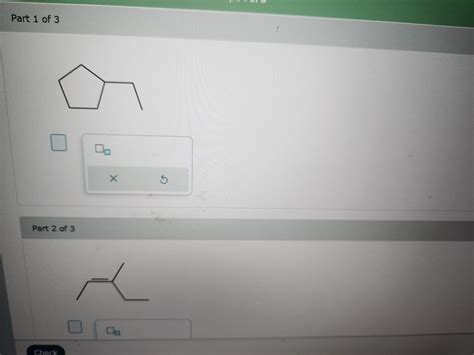
Table of Contents
- Provide The Formula For Each Compound.
- Table of Contents
- The Chemical Formula: A Deep Dive into the Building Blocks of Matter
- What is a Chemical Formula?
- Types of Chemical Formulas
- Examples of Chemical Formulas and their Significance
- The Importance of Correct Chemical Formulas
- Historical Context and the Evolution of Chemical Formulas
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
The Chemical Formula: A Deep Dive into the Building Blocks of Matter
Understanding chemical formulas is fundamental to comprehending the world around us. From the air we breathe to the food we eat, everything is composed of chemical compounds, each with its unique formula representing its constituent elements and their proportions. This comprehensive guide will explore the concept of chemical formulas, delve into various types, and provide examples to solidify your understanding. We will also touch upon the historical context and the importance of correctly interpreting these symbolic representations of matter.
What is a Chemical Formula?
A chemical formula is a concise way of representing the atoms present in a chemical compound or molecule, using element symbols and numerical subscripts. These symbols, derived from the periodic table, provide information about the type and number of atoms of each element within the compound. For instance, H₂O, the formula for water, indicates that a single water molecule contains two hydrogen (H) atoms and one oxygen (O) atom.
Types of Chemical Formulas
Several types of chemical formulas exist, each providing varying levels of detail about a compound's structure:
1. Empirical Formula: This formula represents the simplest whole-number ratio of atoms in a compound. It doesn't necessarily show the actual number of atoms present in a molecule, only their relative proportions. For example, the empirical formula for glucose (C₆H₁₂O₆) is CH₂O, indicating a 1:2:1 ratio of carbon, hydrogen, and oxygen atoms.
2. Molecular Formula: This formula shows the actual number of each type of atom present in a molecule of a compound. It provides a complete picture of the molecular composition. For glucose, the molecular formula is C₆H₁₂O₆, accurately reflecting the six carbon, twelve hydrogen, and six oxygen atoms in each molecule.
3. Structural Formula: This formula goes beyond simply showing the types and numbers of atoms; it illustrates the arrangement of atoms within the molecule. It uses lines to represent bonds between atoms, revealing the molecule's connectivity and spatial structure. Structural formulas are particularly useful for organic compounds with complex structures.
4. Condensed Structural Formula: This is a simplified version of the structural formula, where some bonds and/or atoms are implied rather than explicitly drawn. This makes it more compact while still conveying the essential structural information.
5. Skeletal Formula (Line-Angle Formula): This is a highly simplified representation, common in organic chemistry, where carbon atoms are implied at the intersections and ends of lines, and hydrogen atoms bonded to carbon are omitted for brevity. Only heteroatoms (atoms other than carbon and hydrogen) are explicitly shown.
Examples of Chemical Formulas and their Significance
Let's explore some common chemical formulas and their significance:
1. Water (H₂O): The most ubiquitous compound, essential for all known forms of life. Its formula reveals its simple composition: two hydrogen atoms covalently bonded to one oxygen atom. The polar nature of the molecule, due to the electronegativity difference between oxygen and hydrogen, is responsible for many of water's unique properties.
2. Carbon Dioxide (CO₂): A vital greenhouse gas, crucial for photosynthesis and the carbon cycle. Its formula indicates one carbon atom double-bonded to two oxygen atoms. The linear structure and polar bonds contribute to its role in climate regulation.
3. Sodium Chloride (NaCl): Common table salt, an ionic compound formed by the electrostatic attraction between sodium (Na⁺) and chloride (Cl⁻) ions. Its formula represents the 1:1 ratio of these ions in the crystalline structure. The ionic bonding accounts for its high melting point and solubility in water.
4. Glucose (C₆H₁₂O₆): A simple sugar, a primary source of energy for living organisms. Its formula shows the six carbon, twelve hydrogen, and six oxygen atoms arranged in a specific ring structure. The many hydroxyl groups (-OH) contribute to its solubility and reactivity.
5. Methane (CH₄): The simplest hydrocarbon, a major component of natural gas. Its formula shows one carbon atom bonded to four hydrogen atoms in a tetrahedral geometry. Its combustion releases significant energy, making it a valuable fuel source.
6. Ethanol (C₂H₅OH): The alcohol found in alcoholic beverages. Its formula indicates two carbon atoms, six hydrogen atoms, and one hydroxyl group (-OH). The presence of the hydroxyl group makes it polar and soluble in water.
7. Ammonia (NH₃): A pungent-smelling gas used in fertilizers and various industrial processes. Its formula indicates one nitrogen atom bonded to three hydrogen atoms in a trigonal pyramidal geometry. The lone pair of electrons on nitrogen makes it a weak base.
8. Sulfuric Acid (H₂SO₄): A highly corrosive strong acid, widely used in industrial processes. Its formula shows two hydrogen atoms, one sulfur atom, and four oxygen atoms. The presence of multiple polar bonds and its ability to donate protons contribute to its high acidity.
9. Table Sugar (Sucrose) (C₁₂H₂₂O₁₁): A disaccharide composed of glucose and fructose. Its formula shows a 12:22:11 ratio of carbon, hydrogen, and oxygen. The glycosidic bond between glucose and fructose determines its properties.
10. Acetic Acid (CH₃COOH): The main component of vinegar, giving it its characteristic sour taste. Its formula highlights the presence of a carboxyl group (-COOH), which is responsible for its acidic properties.
The Importance of Correct Chemical Formulas
Accurate chemical formulas are crucial for several reasons:
-
Stoichiometry: Chemical formulas are essential for performing stoichiometric calculations, which allow us to determine the quantities of reactants and products involved in chemical reactions.
-
Chemical Reactions: Understanding the formulas of reactants is vital for predicting the products and understanding the mechanisms of chemical reactions.
-
Chemical Properties: The formula of a compound reveals information about its chemical properties, such as its acidity, basicity, or reactivity.
-
Chemical Synthesis: Knowing the desired product's formula is essential in designing synthetic routes to produce that compound.
-
Material Science: Chemical formulas are fundamental to understanding the properties and behavior of materials, ranging from polymers to ceramics.
Historical Context and the Evolution of Chemical Formulas
The development of chemical formulas was a gradual process, linked to the advancement of atomic theory and chemical understanding. Early attempts at representing chemical composition were largely qualitative, lacking the precision of modern formulas. John Dalton's atomic theory (early 19th century) provided the foundation for representing compounds using symbols and ratios. Jöns Jakob Berzelius later introduced the system of using element symbols we use today. The development of spectroscopy and other analytical techniques further refined our ability to determine the accurate composition of compounds, leading to the sophisticated formulas used in modern chemistry.
Conclusion
Chemical formulas are the cornerstone of chemical communication, providing a concise and precise way to represent the composition of matter. From simple diatomic molecules to complex organic structures, these formulas encode essential information crucial for understanding chemical behavior, designing chemical reactions, and advancing scientific knowledge. Mastering the interpretation and application of chemical formulas is paramount for anyone seeking to delve deeper into the fascinating world of chemistry. Understanding the various types of formulas and their nuances is key to unlocking a more comprehensive understanding of the chemical world. This detailed exploration provides a solid foundation for further study in chemistry and related fields.
Latest Posts
Latest Posts
-
The Empirical Method Of Study Is Based On
Mar 16, 2025
-
How Many Valence Electrons Does Br Have
Mar 16, 2025
-
El Hijo De Mi Padre Es Mi
Mar 16, 2025
-
Which One Of The Following Statements Is True
Mar 16, 2025
-
Which Is Not A N Expense Account
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about Provide The Formula For Each Compound. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
