Predict The Initial And Isolated Products For The Reaction
Holbox
Mar 22, 2025 · 6 min read
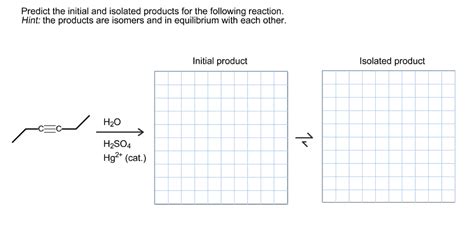
Table of Contents
- Predict The Initial And Isolated Products For The Reaction
- Table of Contents
- Predicting Initial and Isolated Products in Chemical Reactions: A Comprehensive Guide
- Understanding the Difference: Initial vs. Isolated Products
- Factors Influencing Product Formation
- Predicting Products for Different Reaction Types
- Advanced Techniques for Product Prediction
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Predicting Initial and Isolated Products in Chemical Reactions: A Comprehensive Guide
Predicting the products of a chemical reaction is a fundamental skill in chemistry. While seemingly straightforward for simple reactions, predicting the initial and isolated products often requires a deeper understanding of reaction mechanisms, kinetics, and thermodynamics. This article delves into the intricacies of predicting these products, covering various reaction types and highlighting the factors influencing product formation.
Understanding the Difference: Initial vs. Isolated Products
Before diving into prediction methods, it's crucial to distinguish between initial and isolated products.
-
Initial Products: These are the products formed directly from the initial collision or interaction between reactants. They represent the immediate outcome of the reaction before any subsequent transformations occur. These products may be unstable or highly reactive, leading to further reactions.
-
Isolated Products: These are the products that are actually obtained at the end of the reaction, after all subsequent transformations and purifications. They represent the stable, final products of the reaction sequence. These are the products you'd typically isolate and characterize in a laboratory setting.
The difference between initial and isolated products is often significant, particularly in reactions involving unstable intermediates or those that undergo further reactions under the reaction conditions.
Factors Influencing Product Formation
Several factors influence which products are formed, both initially and ultimately:
-
Reactant Nature: The inherent properties of the reactants, such as their functional groups, reactivity, and steric hindrance, heavily influence the reaction pathway and product formation. For example, the presence of electron-withdrawing or electron-donating groups can significantly alter the reactivity of a molecule.
-
Reaction Conditions: Temperature, pressure, solvent, and the presence of catalysts or inhibitors all play a crucial role in determining the reaction pathway and the relative amounts of different products formed. A change in temperature might favor a different reaction mechanism, leading to different products.
-
Reaction Mechanism: The step-by-step process by which a reaction occurs dictates the intermediate and final products. Understanding the reaction mechanism, involving identifying intermediates and transition states, is essential for accurate prediction.
-
Thermodynamics and Kinetics: Thermodynamic considerations determine the relative stability of different products. The most stable products are often favored at equilibrium. However, kinetics play a vital role, as faster reactions might yield kinetically controlled products, even if they're less thermodynamically stable.
-
Stereochemistry: The spatial arrangement of atoms in molecules significantly affects reactivity and product formation. Stereoselective and stereospecific reactions produce specific stereoisomers, making stereochemical considerations crucial for accurate prediction.
Predicting Products for Different Reaction Types
Let's examine product prediction for several common reaction types:
1. Acid-Base Reactions:
Predicting products in acid-base reactions is relatively straightforward. The strongest acid reacts with the strongest base to form a conjugate base and conjugate acid. Consider the reaction between HCl (strong acid) and NaOH (strong base):
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
The initial and isolated products are the same: sodium chloride (NaCl) and water (H₂O).
2. Precipitation Reactions:
In precipitation reactions, the mixing of two aqueous solutions leads to the formation of an insoluble solid (precipitate). Solubility rules help predict whether a precipitate will form. For example, mixing silver nitrate (AgNO₃) and sodium chloride (NaCl) solutions:
AgNO₃(aq) + NaCl(aq) → AgCl(s) + NaNO₃(aq)
The initial and isolated products are silver chloride (AgCl) precipitate and sodium nitrate (NaNO₃) in solution.
3. Redox Reactions:
Redox reactions involve electron transfer. Predicting products requires determining the oxidation states of the reactants and identifying the oxidizing and reducing agents. Consider the reaction between zinc (Zn) and copper(II) sulfate (CuSO₄):
Zn(s) + CuSO₄(aq) → ZnSO₄(aq) + Cu(s)
Zinc is oxidized (loses electrons), and copper(II) is reduced (gains electrons). The initial and isolated products are zinc sulfate (ZnSO₄) and copper (Cu) metal.
4. Addition Reactions (e.g., electrophilic addition):
Addition reactions involve the addition of atoms or groups to a multiple bond (e.g., double or triple bond). The mechanism of addition (e.g., Markovnikov's rule for electrophilic addition to alkenes) dictates the regioselectivity (where the atoms add). For example, the addition of HBr to propene:
CH₃CH=CH₂ + HBr → CH₃CHBrCH₃ (major product)
The initial product is likely a carbocation intermediate, which rapidly reacts with the bromide ion. The isolated product is 2-bromopropane, following Markovnikov's rule.
5. Substitution Reactions (e.g., SN1 and SN2):
Substitution reactions involve the replacement of an atom or group in a molecule. The mechanism (SN1 or SN2) significantly impacts the products. SN1 reactions often lead to racemization, while SN2 reactions show inversion of stereochemistry. For instance, the reaction between 2-bromobutane and hydroxide ion:
CH₃CHBrCH₂CH₃ + OH⁻ → CH₃CHOHCH₂CH₃ (major product, SN2 reaction)
The isolated product is 2-butanol, but the reaction pathway and stereochemistry depend on the reaction conditions (favoring SN1 or SN2).
6. Elimination Reactions:
Elimination reactions involve the removal of atoms or groups from a molecule, often forming a multiple bond. The type of elimination (E1 or E2) influences the product distribution. The dehydration of alcohols is a typical example:
CH₃CH₂OH → CH₂=CH₂ + H₂O
The isolated product is ethene, and the initial product is likely a carbocation intermediate (in an E1 mechanism).
7. Condensation Reactions:
Condensation reactions involve the joining of two molecules with the loss of a small molecule, such as water. Esterification is a classic example:
CH₃COOH + CH₃CH₂OH → CH₃COOCH₂CH₃ + H₂O
The initial product might involve an intermediate, but the isolated product is ethyl acetate and water.
8. Polymerization Reactions:
Polymerization reactions involve the joining of many small molecules (monomers) to form a large molecule (polymer). The type of polymerization (addition or condensation) dictates the structure of the polymer. For example, the polymerization of ethene to form polyethylene:
n CH₂=CH₂ → [-CH₂-CH₂-]ₙ
The isolated product is polyethylene, a long-chain hydrocarbon.
Advanced Techniques for Product Prediction
For more complex reactions, advanced techniques are necessary:
-
Computational Chemistry: Using computational methods like density functional theory (DFT) and molecular mechanics can provide insights into reaction mechanisms, transition states, and relative energies of different products.
-
Spectroscopic Techniques: Techniques such as NMR, IR, and Mass Spectrometry are invaluable for identifying and characterizing both initial and isolated products.
-
Kinetic Studies: Studying reaction rates can provide information on the reaction mechanism and the relative importance of different pathways.
Conclusion
Predicting the initial and isolated products of a chemical reaction requires a multifaceted understanding of reaction mechanisms, thermodynamics, kinetics, and the influence of reaction conditions. While simple reactions may allow straightforward prediction, more complex reactions often demand the use of advanced techniques and a comprehensive knowledge of chemical principles. The ability to accurately predict reaction outcomes is vital for designing efficient synthetic routes and understanding chemical processes across various fields, from organic synthesis to industrial chemistry. By combining theoretical knowledge with experimental techniques, chemists can unravel the complexities of chemical reactions and accurately predict the products formed. Remember to always prioritize safety and follow appropriate laboratory procedures when conducting chemical experiments.
Latest Posts
Latest Posts
-
Which Of The Following Best Describes Angina Pectoris
Mar 24, 2025
-
Only A Person Could Believe Her Tale
Mar 24, 2025
-
Which Statement About Schizophrenia Is True
Mar 24, 2025
-
What Is The Adjective Form Of Heart
Mar 24, 2025
-
Histamine Serotonin And Bradykinin Are All
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Predict The Initial And Isolated Products For The Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
