Nanh2 Is The Conjugate Base Of
Holbox
Mar 18, 2025 · 5 min read
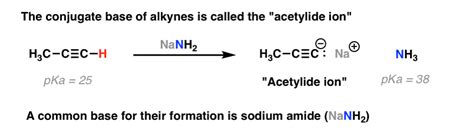
Table of Contents
NANH2 is the Conjugate Base of: Understanding Acid-Base Chemistry
Understanding conjugate acid-base pairs is fundamental to grasping acid-base chemistry. This article delves deep into the concept, focusing specifically on NANH2 and identifying its conjugate acid. We'll explore the Brønsted-Lowry theory, examine the properties of NANH2, and clarify its role in various chemical reactions. We will also touch upon the implications of its strong basicity and its use in organic chemistry.
Understanding the Brønsted-Lowry Theory
The Brønsted-Lowry theory defines acids and bases based on proton (H⁺) transfer. An acid is a substance that donates a proton, while a base is a substance that accepts a proton. Crucially, this theory introduces the concept of conjugate acid-base pairs. When an acid donates a proton, it forms its conjugate base. Conversely, when a base accepts a proton, it forms its conjugate acid. These pairs are related by the difference of a single proton.
Key takeaway: Conjugate acid-base pairs differ only by a single proton (H⁺).
Identifying the Conjugate Acid of NANH2
NANH2, sodium amide, is a strong base. To identify its conjugate acid, we need to consider what happens when it accepts a proton. The reaction would look like this:
NANH2 + H⁺ ⇌ NH3 + Na⁺
In this reaction, NANH2 (sodium amide) accepts a proton (H⁺) to form ammonia (NH3) and a sodium cation (Na⁺). Therefore, the conjugate acid of NANH2 is NH3 (ammonia).
Properties of NANH2 (Sodium Amide)
NANH2, or sodium amide, is a highly reactive and strong base. Its properties are crucial to understanding its role in chemical reactions and its relationship with its conjugate acid, ammonia.
-
Strong Basicity: NANH2 readily deprotonates even weak acids. This strong basicity stems from the highly electronegative nitrogen atom and the presence of the sodium cation, which stabilizes the negative charge on the amide ion (NH2⁻).
-
Reactivity with Water: NANH2 reacts violently with water, undergoing a hydrolysis reaction:
NANH2 + H₂O → NH₃ + NaOH
This reaction generates ammonia and sodium hydroxide, highlighting its sensitivity to moisture. This characteristic necessitates handling NANH2 under anhydrous (water-free) conditions.
-
Use as a Dehydrating Agent: Due to its strong affinity for protons, NANH2 can act as a powerful dehydrating agent, removing water molecules from other compounds.
-
Applications in Organic Chemistry: NANH2 is a versatile reagent in organic synthesis. It's commonly used in reactions requiring strong bases, such as:
- Deprotonation of terminal alkynes: Forming acetylide ions.
- Formation of amides: Reacting with alkyl halides.
- Reductive amination: Converting ketones or aldehydes into amines.
- Birch reduction: A specific type of reduction reaction used in organic synthesis.
Properties of NH3 (Ammonia) – The Conjugate Acid
Ammonia (NH3), the conjugate acid of NANH2, possesses a contrasting set of properties compared to its conjugate base.
-
Weak Basicity: While NH3 is a base, it's significantly weaker than NANH2. It accepts protons but less readily.
-
Gaseous State: Ammonia is a gas at room temperature, unlike the solid sodium amide.
-
Water Solubility: Ammonia is highly soluble in water, forming an alkaline solution (ammonium hydroxide).
-
Versatile Applications: Ammonia has numerous applications, including:
- Fertilizer production: A crucial component in nitrogen-based fertilizers.
- Refrigerant: Used in refrigeration systems.
- Cleaning agent: Found in various household cleaners.
- Production of other chemicals: A precursor for numerous chemicals including nitric acid.
The Acid-Base Equilibrium: NANH2 and NH3
The relationship between NANH2 and NH3 is an equilibrium reaction, represented as:
NH3 ⇌ H⁺ + NH₂⁻
The position of this equilibrium heavily favors the formation of NH3 in the presence of a proton source. The addition of a strong acid will shift the equilibrium to the left, favoring the formation of NH3. Conversely, the addition of a strong base will shift the equilibrium to the right, forming more NANH2. This dynamic equilibrium highlights the interplay between conjugate acid-base pairs.
Practical Implications and Applications
Understanding the conjugate acid-base relationship between NANH2 and NH3 has significant implications in various fields, particularly in:
-
Organic Chemistry Synthesis: As previously mentioned, NANH2's strong basicity makes it a crucial reagent for a variety of organic transformations. Its ability to deprotonate weak acids is vital for creating reactive intermediates. Knowing its conjugate acid helps chemists predict reaction pathways and optimize reaction conditions.
-
Inorganic Chemistry: The reaction between NANH2 and water, forming ammonia and sodium hydroxide, is an important concept in inorganic chemistry. This reaction highlights the reactivity of strong bases and the importance of anhydrous conditions in handling such reagents.
-
Industrial Processes: The industrial synthesis of ammonia (Haber-Bosch process) is a cornerstone of modern agriculture. Understanding the properties of both ammonia and its conjugate base, NANH2, is essential for optimizing this crucial process.
-
Environmental Science: Ammonia's role in the nitrogen cycle is significant in understanding environmental processes. The release of ammonia into the environment can have various ecological consequences, impacting water quality and soil fertility.
-
Analytical Chemistry: The acid-base properties of ammonia and sodium amide are utilized in titrations and other analytical techniques to determine the concentration of acids or bases in a solution.
Safety Precautions
Handling NANH2 requires extreme caution due to its reactivity with water and air. It should be handled under strictly anhydrous conditions, using inert atmospheres like nitrogen or argon. Appropriate personal protective equipment (PPE) including gloves, goggles, and lab coats is mandatory when working with sodium amide. Contact with skin or eyes can cause severe burns.
Conclusion: The Importance of Conjugate Acid-Base Pairs
The concept of conjugate acid-base pairs is central to understanding acid-base chemistry. NANH2 and NH3 serve as a powerful example of this relationship. Knowing that NANH2 is the conjugate base of NH3 allows us to predict its reactivity, understand its applications, and handle it safely. The strong basicity of NANH2 and the weaker basicity of NH3 highlight the significant differences between a compound and its conjugate, illustrating the importance of proton transfer in acid-base reactions. Understanding these fundamental concepts is essential for anyone working in chemistry, whether in academia, industry, or related fields. This knowledge underpins a wide range of chemical processes and applications. From organic synthesis to industrial processes, the conjugate acid-base pair of NANH2 and NH3 plays a vital role.
Latest Posts
Latest Posts
-
Within Mindbridge What Is A Control Point
Mar 18, 2025
-
The Percent Frequency Of A Class Is Computed By
Mar 18, 2025
-
Under What Circumstances Should A Companys Management Team
Mar 18, 2025
-
Who Can Apply Pesticides In A Food Service Establishment
Mar 18, 2025
-
A Nation Can Increase Its Production Possibilities By
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Nanh2 Is The Conjugate Base Of . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
