Mono Addition Of Hbr To Unsymmetrical Dienes
Holbox
Mar 30, 2025 · 5 min read
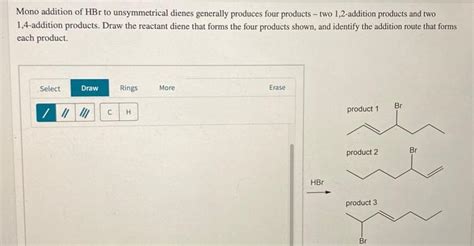
Table of Contents
- Mono Addition Of Hbr To Unsymmetrical Dienes
- Table of Contents
- Mono Addition of HBr to Unsymmetrical Dienes: A Comprehensive Guide
- Understanding the Reaction: 1,2- vs 1,4-Addition
- Reaction Mechanism: A Deep Dive
- 1,2-Addition Mechanism:
- 1,4-Addition (Conjugate Addition) Mechanism:
- Regioselectivity: Markovnikov's Rule and Beyond
- Factors Influencing Product Distribution: Temperature and Solvent Effects
- Temperature Effects:
- Solvent Effects:
- Practical Applications and Significance
- Beyond HBr: Other Hydrogen Halides
- Conclusion: A Summary and Future Directions
- Latest Posts
- Latest Posts
- Related Post
Mono Addition of HBr to Unsymmetrical Dienes: A Comprehensive Guide
The addition of hydrogen halides, particularly HBr, to unsaturated systems is a fundamental reaction in organic chemistry. While the addition to symmetrical alkenes is straightforward, the reaction with unsymmetrical dienes introduces an element of complexity due to the possibility of multiple addition products. This article delves into the intricacies of mono addition of HBr to unsymmetrical dienes, exploring the reaction mechanism, regioselectivity, and factors influencing the product distribution. We'll also examine the application of Markovnikov's rule and its limitations in this context.
Understanding the Reaction: 1,2- vs 1,4-Addition
Unsymmetrical dienes possess two distinct double bonds, each with its own reactivity. When reacting with HBr, the possibility of 1,2- and 1,4-addition arises.
-
1,2-Addition: The HBr adds across one double bond only, forming a product where the hydrogen and bromine are added to adjacent carbon atoms. This is a direct addition, akin to the addition of HBr to a simple alkene.
-
1,4-Addition (Conjugate Addition): The HBr adds across the conjugated diene system, with the hydrogen attaching to one terminal carbon and the bromine to the other terminal carbon, resulting in a resonance-stabilized intermediate. This process involves the formation of an allylic carbocation intermediate, followed by bromide ion attack.
The relative proportions of 1,2- and 1,4-addition products are heavily influenced by several factors which we will explore in detail below.
Reaction Mechanism: A Deep Dive
The mechanism of HBr addition to unsymmetrical dienes is multifaceted and depends on the reaction conditions. Let's break down the mechanism for both 1,2- and 1,4-addition:
1,2-Addition Mechanism:
-
Electrophilic Attack: The electrophilic hydrogen atom of HBr attacks the electron-rich double bond. This step is similar to the addition of HBr to a simple alkene. The pi electrons from the double bond shift to form a bond with the hydrogen, generating a carbocation.
-
Nucleophilic Attack: The bromide ion, a nucleophile, attacks the positively charged carbocation. This results in the formation of the 1,2-addition product.
1,4-Addition (Conjugate Addition) Mechanism:
-
Electrophilic Attack: Again, the electrophilic hydrogen of HBr attacks a double bond, but this time, the attack is more nuanced. The pi electrons in the conjugated system can delocalize, creating resonance-stabilized allylic carbocations. The electrophilic attack preferentially occurs at the carbon that leads to a more stable resonance-stabilized allylic cation.
-
Resonance Stabilization: The resulting allylic carbocation is stabilized by resonance. This resonance stabilization is key to understanding the prevalence of 1,4-addition under certain conditions.
-
Nucleophilic Attack: The bromide ion attacks either terminal carbon of the allylic carbocation. Attack at the terminal carbon, furthest from the initial proton addition, leads to the 1,4-addition product.
Regioselectivity: Markovnikov's Rule and Beyond
Markovnikov's rule, which states that the hydrogen atom adds to the carbon atom with more hydrogen atoms, generally governs the regioselectivity of electrophilic addition reactions to alkenes. However, its applicability to the mono addition of HBr to unsymmetrical dienes is not straightforward.
While the initial protonation step may follow a Markovnikov-like pattern, the subsequent nucleophilic attack on the resonance-stabilized allylic cation introduces further complexity. The relative stability of the resonance contributors, steric effects, and the presence of solvents all contribute to the ultimate regioselectivity.
Factors Influencing Product Distribution: Temperature and Solvent Effects
The reaction conditions play a pivotal role in determining the ratio of 1,2- and 1,4-addition products.
Temperature Effects:
At lower temperatures, the kinetic product, typically the 1,2-addition product, is favoured. This is because the 1,2-addition pathway has a lower activation energy and proceeds faster. The less stable carbocation forms rapidly, preventing extensive resonance stabilization and 1,4-addition.
At higher temperatures, the thermodynamic product, usually the 1,4-addition product, dominates. At higher temperatures, the reaction has sufficient energy to overcome the activation barriers for the more stable product, resulting in a higher proportion of the 1,4-addition product. The more stable product results from equilibration of the resonance-stabilized intermediate.
Solvent Effects:
The choice of solvent can significantly affect the reaction pathway. Polar protic solvents stabilize the carbocation intermediates, favoring 1,4-addition. Non-polar solvents, on the other hand, can influence the regioselectivity by affecting the stability of the transition state, leading to either increased 1,2 or 1,4 addition depending on the diene's structure.
Practical Applications and Significance
The addition of HBr to unsymmetrical dienes is not merely a theoretical exercise; it holds practical importance in various chemical syntheses. Understanding the regioselectivity and controlling the product distribution are vital for designing efficient synthetic routes to target molecules.
The products obtained from this reaction can serve as valuable intermediates in the synthesis of more complex organic molecules. The resulting halohydrins and allylic halides find applications in various areas, including pharmaceuticals, polymers, and materials science. Furthermore, this reaction provides an excellent platform for students to deepen their understanding of reaction mechanisms, kinetics and thermodynamics in organic chemistry.
Beyond HBr: Other Hydrogen Halides
While this article focuses primarily on HBr, it's crucial to acknowledge that other hydrogen halides (HCl, HI) can also react with unsymmetrical dienes. However, their reactivity and the resulting product distributions can differ significantly due to the varying electronegativity and steric factors of the halide ions. Generally, HBr is the most commonly studied due to its balance of reactivity and ease of handling compared to HI. HCl's reactivity is lower and may require different reaction conditions.
Conclusion: A Summary and Future Directions
The mono addition of HBr to unsymmetrical dienes is a complex reaction offering a fascinating illustration of reaction mechanisms, regioselectivity, and the interplay between kinetics and thermodynamics. Understanding the influence of temperature, solvent, and the inherent reactivity of the diene itself is essential for predicting and controlling the product distribution.
Further research focusing on the development of new catalysts and reaction conditions to enhance selectivity and efficiency in these reactions is ongoing. This research aims to optimize existing processes and unlock new synthetic possibilities. The field continues to evolve, promising new discoveries and applications in the future.
This comprehensive exploration of the reaction allows for a deeper appreciation of its complexity and significance within the broader field of organic chemistry. This knowledge serves as a valuable tool for synthetic chemists seeking to design efficient and selective routes to diverse organic molecules. The reaction also highlights the importance of considering reaction conditions and mechanistic details for effective outcome prediction and optimization.
Latest Posts
Latest Posts
-
New Design And Production Techniques Have
Apr 01, 2025
-
Match Each Respiratory Volume To Its Definition
Apr 01, 2025
-
The Biggest Disadvantage Of The Sole Proprietorship Is
Apr 01, 2025
-
Correctly Label The Posterior Muscles Of The Thigh
Apr 01, 2025
-
A Processing Department Is An Organization Unit
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Mono Addition Of Hbr To Unsymmetrical Dienes . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
