Molar Mass Of Ba Oh 2
Holbox
Mar 20, 2025 · 6 min read
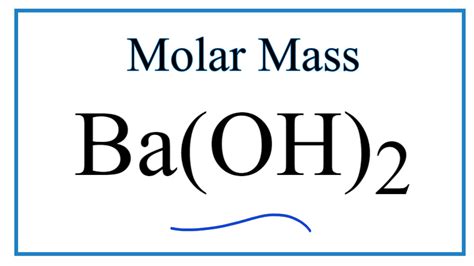
Table of Contents
Molar Mass of Ba(OH)₂: A Deep Dive into Calculation and Applications
The molar mass of barium hydroxide, Ba(OH)₂, is a fundamental concept in chemistry with broad applications in various fields. Understanding its calculation and significance is crucial for accurate stoichiometric calculations, solution preparation, and many analytical procedures. This comprehensive guide will delve into the details of determining the molar mass of Ba(OH)₂, its practical applications, and related concepts.
Understanding Molar Mass
Before we calculate the molar mass of Ba(OH)₂, let's establish a clear understanding of the term. Molar mass refers to the mass of one mole of a substance. A mole is a fundamental unit in chemistry representing Avogadro's number (approximately 6.022 x 10²³) of particles (atoms, molecules, ions, etc.). The molar mass is expressed in grams per mole (g/mol). It essentially acts as a conversion factor between the mass of a substance and the number of moles.
Calculating the Molar Mass of Ba(OH)₂
To calculate the molar mass of Ba(OH)₂, we need to consider the atomic masses of its constituent elements: barium (Ba), oxygen (O), and hydrogen (H). These atomic masses are typically found on the periodic table.
- Barium (Ba): The atomic mass of barium is approximately 137.33 g/mol.
- Oxygen (O): The atomic mass of oxygen is approximately 16.00 g/mol.
- Hydrogen (H): The atomic mass of hydrogen is approximately 1.01 g/mol.
Now, let's break down the calculation:
-
Barium (Ba): There's one barium atom in Ba(OH)₂, so its contribution to the molar mass is 137.33 g/mol.
-
Oxygen (O): There are two oxygen atoms in Ba(OH)₂, so their combined contribution is 2 * 16.00 g/mol = 32.00 g/mol.
-
Hydrogen (H): There are two hydrogen atoms in Ba(OH)₂, so their combined contribution is 2 * 1.01 g/mol = 2.02 g/mol.
-
Total Molar Mass: To obtain the total molar mass of Ba(OH)₂, we sum up the contributions from each element: 137.33 g/mol + 32.00 g/mol + 2.02 g/mol = 171.35 g/mol.
Therefore, the molar mass of barium hydroxide, Ba(OH)₂, is approximately 171.35 g/mol. This value might slightly vary depending on the source of atomic mass data used.
Significance and Applications of Molar Mass of Ba(OH)₂
The molar mass of Ba(OH)₂ is a cornerstone for numerous chemical calculations and applications, including:
1. Stoichiometric Calculations:
In stoichiometry, we use balanced chemical equations to relate the amounts of reactants and products in a chemical reaction. The molar mass acts as a bridge between the mass of a substance and the number of moles involved in the reaction. For instance, if we know the mass of Ba(OH)₂ reacting with another substance, we can use its molar mass to convert that mass into moles and subsequently determine the moles of other reactants or products involved based on the stoichiometric ratios in the balanced equation.
2. Solution Preparation:
Preparing solutions of a specific concentration often requires accurate measurements. Knowing the molar mass of Ba(OH)₂ allows us to precisely calculate the mass required to prepare a solution of a given molarity (moles per liter). For example, to prepare 1 liter of a 0.1 M Ba(OH)₂ solution, we would calculate the required mass using the molar mass: (0.1 mol/L) * (171.35 g/mol) * (1 L) = 17.135 g.
3. Titrations:
Titrations are analytical techniques used to determine the concentration of an unknown solution using a solution of known concentration. The molar mass of Ba(OH)₂ plays a crucial role in titrations involving barium hydroxide as either the titrant or the analyte. The stoichiometry of the reaction between Ba(OH)₂ and the analyte, along with the molar mass of Ba(OH)₂, allows for the precise determination of the unknown concentration.
4. Chemical Analysis:
In various analytical techniques, such as gravimetric analysis, the molar mass of Ba(OH)₂ is essential for converting the measured mass of a precipitate (e.g., barium sulfate formed from a reaction with Ba(OH)₂) into the amount of analyte originally present.
5. Industrial Applications:
Barium hydroxide finds applications in several industries, including:
- Sugar refining: It is used to refine sugar, removing impurities.
- Chemical synthesis: It serves as a starting material in various chemical syntheses.
- Wastewater treatment: It helps to neutralize acidic waste streams.
- Rubber industry: It's used in rubber processing as a vulcanization agent.
- Manufacturing of glass and ceramics: Used as a fluxing agent.
In all these industrial processes, a precise understanding and utilization of the molar mass of Ba(OH)₂ ensure efficient and accurate procedures.
Related Concepts and Calculations
Several related concepts are essential to fully grasp the significance and usage of the molar mass of Ba(OH)₂:
1. Percent Composition:
The percent composition of a compound provides the relative mass percentage of each element present. For Ba(OH)₂, we can calculate the percentage composition as follows:
- % Ba: (137.33 g/mol / 171.35 g/mol) * 100% ≈ 80.1%
- % O: (32.00 g/mol / 171.35 g/mol) * 100% ≈ 18.7%
- % H: (2.02 g/mol / 171.35 g/mol) * 100% ≈ 1.2%
These percentages represent the relative contributions of each element to the overall mass of Ba(OH)₂.
2. Empirical Formula and Molecular Formula:
The empirical formula represents the simplest whole-number ratio of atoms in a compound, while the molecular formula indicates the actual number of atoms in a molecule. For Ba(OH)₂, the empirical and molecular formulas are the same: Ba(OH)₂.
3. Hydrates:
Barium hydroxide can also exist as a hydrate, which means it incorporates water molecules in its crystalline structure. For example, Ba(OH)₂·8H₂O is barium hydroxide octahydrate. Calculating the molar mass of a hydrate requires including the molar mass of the water molecules bound to the barium hydroxide.
4. Molarity and Molality:
Molarity (M) expresses the concentration of a solution in moles of solute per liter of solution, while molality (m) represents moles of solute per kilogram of solvent. The molar mass of Ba(OH)₂ is crucial in converting between mass, moles, and concentration when dealing with solutions.
Conclusion
The molar mass of Ba(OH)₂, approximately 171.35 g/mol, is a vital parameter in various chemical calculations and applications. Understanding its calculation, significance, and its relationship with other crucial concepts like stoichiometry, solution preparation, and chemical analysis is essential for students and professionals in chemistry and related fields. Its role extends beyond academic exercises, finding practical application in diverse industrial processes and analytical techniques. This detailed explanation provides a solid foundation for anyone seeking a comprehensive understanding of this fundamental chemical concept. The information presented here emphasizes the practical significance of molar mass calculations in solving real-world problems involving barium hydroxide and similar compounds. Remember always to double-check your sources for the most up-to-date atomic masses for the most accurate calculations.
Latest Posts
Latest Posts
-
Using Models To Predict Molecular Structure Lab
Mar 21, 2025
-
Based On The Cell Values In Cells B77
Mar 21, 2025
-
Payroll Deductions Are Recorded In A Separate
Mar 21, 2025
-
Insert The Picture File Remodel Jpg As The Worksheet Background
Mar 21, 2025
-
C A Mo Se Llama El Padre De Sara
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Molar Mass Of Ba Oh 2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
