Using Models To Predict Molecular Structure Lab
Holbox
Mar 21, 2025 · 7 min read
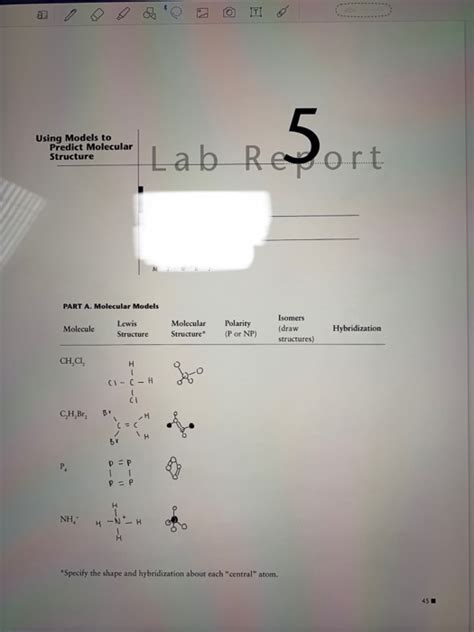
Table of Contents
- Using Models To Predict Molecular Structure Lab
- Table of Contents
- Using Models to Predict Molecular Structure: A Deep Dive into Lab Applications
- The Power of Predictive Modeling in Molecular Structure Determination
- Types of Molecular Modeling Techniques for Structure Prediction
- 1. Molecular Mechanics (MM)
- 2. Quantum Mechanics (QM)
- 3. Hybrid QM/MM Methods
- 4. Machine Learning (ML)
- Practical Considerations for Using Models in the Lab
- 1. Choosing the Appropriate Method
- 2. Force Field Selection (for MM)
- 3. Basis Set Selection (for QM)
- 4. Validation and Verification
- 5. Data Preprocessing and Preparation
- Case Studies: Real-World Applications of Molecular Structure Prediction
- The Future of Molecular Structure Prediction
- Conclusion: Empowering Lab Research with Predictive Modeling
- Latest Posts
- Latest Posts
- Related Post
Using Models to Predict Molecular Structure: A Deep Dive into Lab Applications
Predicting molecular structure is a cornerstone of modern chemistry and materials science. Understanding the three-dimensional arrangement of atoms within a molecule is crucial for predicting its properties, reactivity, and ultimately, its function. Traditional methods for determining molecular structure, such as X-ray crystallography and NMR spectroscopy, are time-consuming, expensive, and sometimes limited in their applicability. This is where computational modeling steps in, offering a powerful and increasingly accurate approach to predicting molecular structures in the lab setting.
The Power of Predictive Modeling in Molecular Structure Determination
Computational models offer several significant advantages over traditional experimental techniques:
-
Speed and Efficiency: Models can predict structures significantly faster than experimental methods, accelerating the research process. This is particularly crucial in drug discovery and materials science where time is a critical factor.
-
Cost-Effectiveness: While initial investment in computational resources might be required, the overall cost of modeling is often lower than experimental techniques, especially for complex molecules or large datasets.
-
Accessibility: Modeling tools are becoming increasingly accessible, empowering researchers with limited resources to perform sophisticated structure predictions.
-
Complementary Approach: Models can complement experimental data, providing insights into structural features that might be challenging to obtain experimentally. They can also help in designing and optimizing experiments.
-
Handling Intractable Systems: Some systems are inherently difficult or impossible to study experimentally (e.g., highly reactive species, transient intermediates). Models provide a valuable means to investigate these systems.
Types of Molecular Modeling Techniques for Structure Prediction
Several computational techniques are used to predict molecular structures, each with its strengths and limitations:
1. Molecular Mechanics (MM)
MM methods use classical physics principles to calculate the energy of a molecule based on its atom positions and predefined force fields. These methods are computationally inexpensive and suitable for large systems but lack the ability to describe bond breaking and formation. They are excellent for optimizing geometries of known structures or for conformational analysis.
Applications in the Lab: MM is frequently used for:
- Conformational analysis: Determining the most stable three-dimensional arrangement of a molecule.
- Molecular docking: Predicting how a molecule (e.g., drug) will bind to a target (e.g., protein).
- Molecular dynamics simulations: Simulating the movement of atoms over time to study dynamic processes.
2. Quantum Mechanics (QM)
QM methods use the principles of quantum mechanics to calculate the electronic structure of molecules. These methods are more accurate than MM but computationally more expensive, limiting their application to smaller systems. QM methods can accurately describe bond breaking and formation, making them suitable for studying chemical reactions.
Different levels of QM theory exist, including:
- Hartree-Fock (HF): A relatively simple method that provides a reasonable starting point but neglects electron correlation.
- Density Functional Theory (DFT): A widely used method that includes electron correlation effects at a relatively low computational cost. It's a powerful workhorse for many applications.
- Post-Hartree-Fock methods (e.g., MP2, CCSD(T)): These methods provide higher accuracy than DFT but are significantly more computationally expensive.
Applications in the Lab: QM methods are invaluable for:
- Reaction mechanism studies: Determining the step-by-step process of a chemical reaction.
- Spectroscopic property prediction: Predicting the vibrational frequencies, NMR chemical shifts, and other spectroscopic properties of a molecule.
- Calculating reaction energies and activation barriers: Understanding the thermodynamics and kinetics of chemical reactions.
3. Hybrid QM/MM Methods
These methods combine the strengths of both QM and MM approaches. A smaller, chemically relevant part of the system (e.g., the reaction center) is treated with QM, while the rest of the system is treated with MM. This approach allows for the study of large systems with high accuracy for the region of interest.
Applications in the Lab: Hybrid QM/MM is particularly useful for:
- Enzyme catalysis: Studying the mechanism of enzyme-catalyzed reactions.
- Protein-ligand interactions: Investigating the interactions between proteins and small molecules.
- Solid-state chemistry: Simulating reactions on surfaces or within crystals.
4. Machine Learning (ML)
ML methods are increasingly used for predicting molecular properties, including structure. These methods learn from large datasets of existing molecular structures and properties and can then predict the properties of new molecules. ML offers the potential for high throughput screening and the discovery of novel materials.
Different ML algorithms are used, including:
- Neural networks: Powerful algorithms that can learn complex relationships in data.
- Support vector machines (SVM): Effective for classification and regression tasks.
- Random forests: Ensemble methods that combine multiple decision trees for improved accuracy.
Applications in the Lab: ML is revolutionizing:
- High-throughput screening: Predicting the properties of large numbers of molecules rapidly.
- Inverse design: Designing molecules with specific properties.
- Accelerating experimental design: Guiding the synthesis and characterization of new molecules.
Practical Considerations for Using Models in the Lab
While modeling offers many advantages, several factors must be considered for successful application:
1. Choosing the Appropriate Method
The choice of modeling method depends on the specific problem, the size of the system, and the desired level of accuracy. A balance between accuracy and computational cost is essential.
2. Force Field Selection (for MM)
The accuracy of MM calculations depends heavily on the choice of force field. Different force fields are parameterized for different types of molecules and may perform better or worse depending on the specific system.
3. Basis Set Selection (for QM)
The choice of basis set in QM calculations affects the accuracy and computational cost. Larger basis sets generally provide higher accuracy but are more computationally expensive.
4. Validation and Verification
It is crucial to validate the models against experimental data or high-level calculations. This helps ensure that the model is accurate and reliable. Blind prediction sets are essential for proper model evaluation.
5. Data Preprocessing and Preparation
Accurate input data is crucial for reliable predictions. This includes proper molecular geometry optimization and cleaning of input structures.
Case Studies: Real-World Applications of Molecular Structure Prediction
Several examples highlight the impact of molecular modeling on various fields:
1. Drug Discovery: Models are used to predict the binding affinity of drug candidates to target proteins, accelerating the drug discovery process and reducing the cost and time required for experimental screening.
2. Materials Science: Models are crucial in the design of new materials with specific properties, such as high strength, conductivity, or catalytic activity. Predicting the structure helps understand these material properties.
3. Environmental Science: Modeling helps predict the behavior of pollutants in the environment, assisting in developing strategies for pollution remediation.
4. Biochemistry: Models are used to study the structure and function of proteins, enzymes, and other biological molecules, leading to a deeper understanding of biological processes.
5. Supramolecular Chemistry: The self-assembly of complex architectures is often modeled to predict the final structure and potentially to direct the synthesis.
The Future of Molecular Structure Prediction
The field of molecular modeling is constantly evolving, with ongoing developments in both methodology and computational power. Advances in machine learning are poised to revolutionize structure prediction, enabling the rapid and accurate prediction of the structures of increasingly complex molecules. The integration of experimental and computational techniques will become increasingly important, with models guiding experiments and experiments validating models. This synergy will drive further progress in understanding and controlling molecular structure, paving the way for significant breakthroughs in chemistry, materials science, and beyond. The use of high-performance computing (HPC) and cloud computing will also play a crucial role in accelerating these advancements.
Conclusion: Empowering Lab Research with Predictive Modeling
Predictive modeling of molecular structures is no longer a niche technique; it’s a powerful tool readily available to researchers across many disciplines. By carefully selecting the appropriate modeling method, validating results, and understanding the limitations of each technique, researchers can leverage the power of computational modeling to significantly enhance their laboratory investigations. The future of scientific discovery increasingly relies on the synergy between experimental approaches and computational modeling, driving advancements that will shape the world around us.
Latest Posts
Latest Posts
-
Which Of The Following Is True Of Blood Vessel Length
Mar 28, 2025
-
When Evaluating The Links In A Message
Mar 28, 2025
-
Estimate The Value Of Each Of The Following
Mar 28, 2025
-
It Is Best Practice To Make Sure Your Data
Mar 28, 2025
-
If A Company Invests In Production Improvement Option D
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Using Models To Predict Molecular Structure Lab . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
