Match Each Definition To The Level Of Protein Structure
Holbox
Mar 17, 2025 · 7 min read
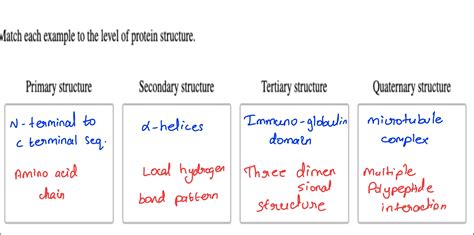
Table of Contents
Matching Definitions to Levels of Protein Structure: A Comprehensive Guide
Proteins are the workhorses of the cell, carrying out a vast array of functions crucial for life. Their ability to perform these diverse roles stems from their intricate three-dimensional structures, which arise from a hierarchy of organizational levels. Understanding these levels – primary, secondary, tertiary, and quaternary structure – is fundamental to grasping protein function and dysfunction. This comprehensive guide will delve into each level, providing clear definitions and examples to solidify your understanding. We'll also explore how deviations from these structures can lead to disease.
Primary Structure: The Linear Sequence of Amino Acids
The primary structure of a protein is its simplest yet most fundamental level. It's defined as the linear sequence of amino acids linked together by peptide bonds. This sequence is dictated by the genetic code, specifically the order of nucleotides in the gene encoding the protein. Think of it as the "alphabet" of the protein world, where each amino acid is a letter. The specific sequence determines all subsequent levels of structure and ultimately, the protein's function.
Key characteristics of primary structure:
- Peptide bonds: These covalent bonds form between the carboxyl group (-COOH) of one amino acid and the amino group (-NH2) of the next, releasing a water molecule. This creates the polypeptide backbone.
- Amino acid sequence: The order of amino acids is crucial. A single change in the sequence (a mutation) can drastically alter the protein's structure and function. This is exemplified by sickle cell anemia, caused by a single amino acid substitution in hemoglobin.
- N-terminus and C-terminus: The polypeptide chain has a free amino group at one end (N-terminus) and a free carboxyl group at the other (C-terminus).
Example: The primary structure of a small peptide might be written as: Met-Gly-Ala-Ser-Leu. This indicates the order of methionine (Met), glycine (Gly), alanine (Ala), serine (Ser), and leucine (Leu) amino acids.
The Importance of Primary Structure:
Any alteration in the primary sequence, even a single amino acid change, can significantly affect the final folded structure of the protein. This alteration can lead to loss of function or the formation of dysfunctional proteins implicated in several diseases, emphasizing the crucial role of the primary structure.
Secondary Structure: Local Folding Patterns
Once the primary sequence is established, the polypeptide chain begins to fold into regular, repeating patterns known as secondary structure. These local structures are stabilized primarily by hydrogen bonds between the backbone atoms (carbonyl oxygen and amide hydrogen) of the amino acids. The two most common types of secondary structures are:
Alpha-Helices: Coiled Structures
Alpha-helices are right-handed coiled structures where the polypeptide backbone spirals around a central axis. The hydrogen bonds form between the carbonyl oxygen of one amino acid and the amide hydrogen of the amino acid four residues down the chain. This creates a rod-like structure.
Key characteristics of alpha-helices:
- Right-handed coil: The helix twists to the right.
- Hydrogen bonding: Stabilizing interaction between backbone atoms.
- 3.6 amino acids per turn: This is a characteristic feature of the alpha-helix structure.
- R-groups: The side chains (R-groups) of the amino acids protrude outwards from the helix.
Beta-Sheets: Extended Structures
Beta-sheets (or β-sheets) are formed by extended stretches of the polypeptide chain arranged side by side. Hydrogen bonds form between carbonyl oxygens and amide hydrogens of adjacent polypeptide strands. These strands can be parallel (running in the same direction) or anti-parallel (running in opposite directions).
Key characteristics of beta-sheets:
- Hydrogen bonding: Stabilizes the sheet structure between adjacent strands.
- Parallel and anti-parallel arrangements: These represent two distinct structural configurations.
- Pleated appearance: The sheets exhibit a pleated or corrugated appearance due to the arrangement of amino acids.
- R-groups: Side chains project alternately above and below the plane of the sheet.
Loops and Turns: Connecting Secondary Structures
Between alpha-helices and beta-sheets, we often find loops and turns. These less-ordered regions connect the more structured elements, enabling the overall protein to fold into its three-dimensional shape.
The importance of Secondary Structure:
The secondary structures contribute significantly to the protein's overall stability and its interaction with other molecules. Their regular patterns provide a foundation upon which tertiary structure is built. Disruptions in secondary structures often lead to protein misfolding and loss of function.
Tertiary Structure: The Three-Dimensional Arrangement
The tertiary structure represents the complete three-dimensional arrangement of a polypeptide chain, including all its secondary structural elements. It's a complex structure stabilized by a variety of interactions between amino acid side chains (R-groups). These include:
- Hydrophobic interactions: Nonpolar side chains cluster together in the protein's interior, away from the aqueous environment.
- Hydrogen bonds: Interactions between polar side chains.
- Ionic bonds (salt bridges): Attractions between oppositely charged side chains.
- Disulfide bonds: Covalent bonds between cysteine residues, which significantly stabilize the structure.
Key characteristics of tertiary structure:
- Globular or fibrous: Proteins can be broadly classified as globular (compact, roughly spherical) or fibrous (elongated, fiber-like).
- Active sites: In enzymes, the tertiary structure creates a unique three-dimensional active site where substrate binding and catalysis occur.
- Domains: Many proteins consist of distinct functional units called domains, each folded independently.
The importance of Tertiary Structure:
The tertiary structure dictates the protein's biological function. Its precise three-dimensional shape enables interactions with specific ligands, substrates, or other proteins. Alterations in tertiary structure, often caused by mutations or environmental changes, can lead to protein misfolding and disease. Examples include Alzheimer's and Parkinson's diseases, where protein aggregation plays a significant role.
Quaternary Structure: Assembly of Multiple Polypeptide Chains
Some proteins are composed of two or more polypeptide chains, called subunits. The quaternary structure describes how these subunits associate to form the complete functional protein. The interactions between subunits are similar to those stabilizing tertiary structure, including hydrophobic interactions, hydrogen bonds, ionic bonds, and disulfide bonds.
Key characteristics of quaternary structure:
- Subunits: Independent polypeptide chains.
- Interactions between subunits: Various forces stabilize the quaternary structure.
- Symmetry: Many proteins with quaternary structure exhibit symmetry in the arrangement of subunits.
- Cooperativity: In some proteins, the binding of a ligand to one subunit can influence the binding of a ligand to other subunits, a phenomenon known as cooperativity (as seen in hemoglobin).
The importance of Quaternary Structure:
Quaternary structure allows for the assembly of large, complex proteins with multiple functional sites or enhanced stability. It's critical for the function of many proteins, including enzymes, receptors, and structural proteins. Disruptions in quaternary structure can lead to loss of function and disease.
Connecting Structure and Function: A Case Study of Hemoglobin
Hemoglobin, the oxygen-carrying protein in red blood cells, is an excellent example illustrating the relationship between protein structure and function across all levels.
- Primary structure: The specific sequence of amino acids in each of its four globin subunits determines how it folds.
- Secondary structure: Alpha-helices are prevalent in hemoglobin's structure, contributing to its overall shape.
- Tertiary structure: Each subunit folds into a compact, globular structure with a heme group nestled within, which binds to oxygen.
- Quaternary structure: Four subunits (two alpha and two beta) assemble to form a tetrameric protein, allowing for cooperative oxygen binding.
Mutations in the primary structure of hemoglobin can lead to diseases like sickle cell anemia, drastically altering its tertiary and quaternary structure, and impairing its ability to carry oxygen efficiently.
Conclusion: The Interplay of Protein Structure and Function
The four levels of protein structure—primary, secondary, tertiary, and quaternary—are intricately linked and essential for understanding protein function. The primary sequence dictates the higher-order structures, which in turn determine the protein's three-dimensional shape and its interactions with other molecules. Deviations from these structures, caused by mutations or environmental factors, can lead to protein dysfunction and a variety of diseases. By understanding these levels, we gain critical insights into the intricacies of biological processes and the molecular basis of disease. Further research continues to expand our understanding of these complex and essential molecules.
Latest Posts
Latest Posts
-
A Smishing Scam Can Involve Which Of The Following
Mar 17, 2025
-
Chilling Is Most Commonly Practiced By
Mar 17, 2025
-
The Revenue Recognition Principle States That Revenue
Mar 17, 2025
-
The Space Between Two Neurons Is Called The
Mar 17, 2025
-
Remote Access May Be Permitted For Privileged Functions
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Match Each Definition To The Level Of Protein Structure . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
