Like Zirconium On The Periodic Table
Holbox
Mar 09, 2025 · 6 min read
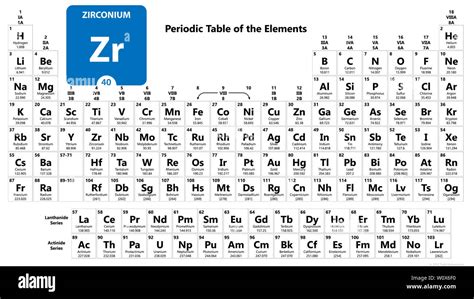
Table of Contents
Like Zirconium on the Periodic Table: A Deep Dive into Element 40
Zirconium, element number 40 on the periodic table, is a fascinating transition metal with a surprisingly rich history and an array of significant applications. While not as famous as some of its neighbors, like titanium or hafnium, understanding zirconium's unique properties and its position within the periodic table unlocks a deeper appreciation for its role in various industries. This comprehensive article will explore zirconium's characteristics, its placement within the periodic table's context, its extraction, its uses, and its future potential.
Zirconium's Place in the Periodic Table: Group 4 and Beyond
Zirconium resides in the fourth group (Group 4) of the periodic table, nestled between titanium (Ti, element 22) and hafnium (Hf, element 72). This grouping signifies several key characteristics shared by these elements:
- Similar Electronic Configuration: All three elements exhibit a similar electron configuration in their outermost shells, leading to comparable chemical behavior. They readily lose electrons to form stable +4 cations.
- Transition Metal Properties: As transition metals, they display properties such as variable oxidation states (though +4 is most common), the ability to form colored compounds, and catalytic activity.
- High Melting Points: Zirconium, titanium, and hafnium boast impressively high melting points, reflecting strong metallic bonding. This robustness is critical in many of their applications.
The lanthanide contraction, a phenomenon where the size of atoms decreases across the lanthanide series, significantly influences zirconium's properties. Hafnium, directly below zirconium, is surprisingly similar in size due to this contraction. This similarity causes significant challenges in separating the two elements, a process crucial for obtaining pure zirconium for specific applications.
Zirconium's Atomic Structure and Properties
Zirconium's atomic number of 40 means it has 40 protons and typically 40 electrons. Its electron configuration is [Kr] 4d² 5s², contributing to its chemical reactivity and bonding characteristics. Key physical and chemical properties include:
- Lustrous, grayish-white metal: In its pure form, zirconium exhibits a bright, metallic luster.
- High melting point (1855 °C): This high melting point makes it suitable for high-temperature applications.
- High strength and durability: Zirconium possesses excellent strength-to-weight ratio.
- Corrosion resistance: A crucial property, zirconium's resistance to corrosion, especially in aqueous environments, is what makes it ideal for various applications.
- Low neutron absorption cross-section: This property is especially important in its use in nuclear reactors.
- Paramagnetic: Zirconium exhibits weak attraction to magnetic fields.
Extracting Zirconium: From Ore to Metal
Zirconium is not found in its elemental form in nature. It's primarily extracted from its ores, the most important of which is zircon (ZrSiO₄). The extraction process involves several stages:
-
Mining and Concentration: Zircon is mined from various locations worldwide, often as a byproduct of other mining operations. The ore is then concentrated to increase the zirconium content.
-
Conversion to Zirconium Dioxide (ZrO₂): Zircon is converted to zirconium dioxide through a process involving chlorination or alkaline fusion. This step is crucial for purifying the zirconium and separating it from other elements like hafnium.
-
Hafnium Separation: Because hafnium's similar size and chemical properties, separating it from zirconium is a significant challenge. This separation is crucial because hafnium has a high neutron absorption cross-section, making it unsuitable for many applications of zirconium. Methods such as liquid-liquid extraction are employed to achieve this separation.
-
Reduction to Metallic Zirconium: The purified zirconium dioxide is then reduced to metallic zirconium. Common methods include the Kroll process, which involves reacting zirconium tetrachloride (ZrCl₄) with magnesium. The resulting zirconium sponge is then refined further.
-
Refining and Fabrication: The final step involves melting, casting, and further refining to achieve the desired purity and form for specific applications.
Diverse Applications of Zirconium: Across Industries
Zirconium's unique blend of properties makes it exceptionally versatile, leading to its use in various industries:
1. Nuclear Industry: A cornerstone of reactor technology
Zirconium's low neutron absorption cross-section makes it an indispensable material in nuclear reactors. Zirconium alloys are used to clad nuclear fuel rods, preventing the release of radioactive materials while allowing for efficient heat transfer. This application highlights the importance of the careful separation of zirconium from hafnium, as the latter's high neutron absorption would significantly hinder reactor efficiency.
2. Chemical Industry: Corrosion resistance in demanding environments
Zirconium's exceptional corrosion resistance makes it ideal for handling corrosive chemicals in the chemical industry. It's used in reaction vessels, pipes, and heat exchangers where other materials would quickly degrade. Its ability to withstand harsh conditions extends its lifespan and reduces maintenance costs.
3. Aerospace Industry: Strength and lightness in demanding conditions
Zirconium alloys are used in aerospace applications where high strength-to-weight ratio is essential. Its ability to withstand extreme temperatures and pressures makes it suitable for components in high-performance aircraft and spacecraft.
4. Medical Implants: Biocompatibility and durability
Zirconium's biocompatibility makes it a suitable material for medical implants. Its corrosion resistance and strength make it appropriate for hip replacements, dental implants, and other medical devices that require long-term stability within the body.
5. Ceramics and Glass Industry: Improving properties
Zirconium oxide (ZrO₂) is used as an additive in ceramics and glass to improve their strength, toughness, and resistance to thermal shock. It is commonly found in high-performance ceramics used in cutting tools and other industrial applications.
6. Other Notable Applications
Zirconium also finds use in a wide range of other applications, including:
- Jewelry: Zirconium is sometimes used as a less expensive alternative to diamonds.
- Superalloys: Small amounts of zirconium are used in superalloys to enhance their properties.
- Flashbulbs: Zirconium was historically used in flashbulbs, but it has been replaced by other materials.
Future Potential of Zirconium: Expanding Horizons
Research and development efforts continue to explore new and exciting applications for zirconium. These include:
- Advanced materials: The development of new zirconium alloys with enhanced properties is ongoing, opening up possibilities for new applications in various high-tech industries.
- Fuel cells: Zirconium-based materials are being investigated for use in fuel cell technology, where their ability to conduct ions at high temperatures is advantageous.
- Renewable energy: Zirconium's properties are being explored for use in solar energy technologies and other renewable energy applications.
- Catalysis: Zirconium-based catalysts are being developed for a variety of chemical reactions.
Conclusion: A Versatile Element with a Bright Future
Zirconium, element 40, occupies a significant place in the periodic table and in modern technology. Its unique blend of properties – high strength, corrosion resistance, low neutron absorption – has led to its widespread use in various industries, from nuclear reactors to medical implants. Ongoing research is continually uncovering new and innovative applications, highlighting the significant potential of this versatile and remarkable element. Its position in the periodic table, alongside titanium and hafnium, perfectly illustrates the trends and complexities of chemical properties within transition metal groups. As we continue to push the boundaries of material science and engineering, zirconium’s role will undoubtedly expand, securing its place as a vital element in shaping our future.
Latest Posts
Latest Posts
-
13 Out Of 16 As A Percentage
Mar 10, 2025
-
Short Happy Life Of Francis Macomber
Mar 10, 2025
-
How To Calculate The Range Of Temperature
Mar 10, 2025
-
How Many Liters Is 55 Gallons
Mar 10, 2025
-
Is Chlorine An Acid Or Base
Mar 10, 2025
Related Post
Thank you for visiting our website which covers about Like Zirconium On The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
