Lewis Structure For Po Oh 3
Holbox
Mar 17, 2025 · 5 min read
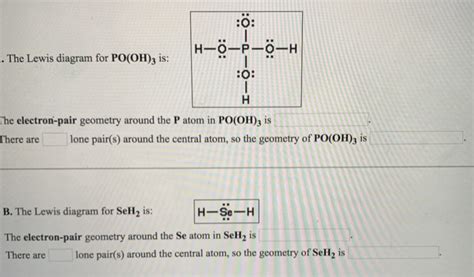
Table of Contents
Lewis Structure for PO(OH)₃: A Comprehensive Guide
The Lewis structure, also known as the Lewis dot diagram, is a crucial tool in chemistry for visualizing the bonding and arrangement of atoms within a molecule. Understanding the Lewis structure is essential for predicting molecular geometry, polarity, and reactivity. This article will delve deeply into constructing the Lewis structure for phosphoric acid, PO(OH)₃, explaining each step in detail and exploring its implications.
Understanding the Basics: What is a Lewis Structure?
A Lewis structure is a simplified representation of a molecule's valence electrons. Valence electrons are the outermost electrons of an atom, and they are the ones involved in chemical bonding. In a Lewis structure, these valence electrons are represented as dots surrounding the atomic symbol. Bonds between atoms are represented by lines, where each line represents a shared pair of electrons (a covalent bond).
Step-by-Step Construction of the PO(OH)₃ Lewis Structure
Let's break down the process of drawing the Lewis structure for phosphoric acid, PO(OH)₃, step-by-step:
Step 1: Determine the Total Number of Valence Electrons
- Phosphorus (P): Phosphorus is in Group 15 (or VA), so it has 5 valence electrons.
- Oxygen (O): There are four oxygen atoms, each contributing 6 valence electrons (4 x 6 = 24).
- Hydrogen (H): There are three hydrogen atoms, each contributing 1 valence electron (3 x 1 = 3).
Total valence electrons: 5 + 24 + 3 = 32
Step 2: Identify the Central Atom
Phosphorus (P) is the least electronegative atom among the others, making it the most likely candidate to be the central atom.
Step 3: Connect Atoms with Single Bonds
Connect the central phosphorus atom to each of the four oxygen atoms with single bonds. This uses 8 electrons (4 bonds x 2 electrons/bond).
Step 4: Distribute Remaining Electrons to Achieve Octet Rule
We have 32 - 8 = 24 electrons remaining. Distribute these electrons to the oxygen atoms, ensuring that each oxygen atom achieves an octet (8 electrons around it). Remember that hydrogen only needs 2 electrons for a stable configuration (duet rule).
Step 5: Check for Octet Rule Satisfaction
At this point, check if all atoms (except hydrogen) have eight electrons surrounding them. If some atoms have fewer than eight electrons, form double or triple bonds to satisfy the octet rule. In the case of PO(OH)₃, the three hydroxyl (-OH) groups already have their oxygen atoms satisfying the octet rule, with two electrons shared with hydrogen and two lone pairs. The additional oxygen, however, will have a single bond with the phosphorus, and needs further electrons to fulfil the octet rule. A double bond between the phosphorus and the additional oxygen atom will solve this problem. This will leave the phosphorus with 10 electrons.
Step 6: Formal Charge Calculation (Optional but Important)
Calculating formal charges helps determine the most stable Lewis structure. The formal charge of an atom is calculated as:
Formal charge = (Valence electrons) - (Non-bonding electrons) - (1/2 * Bonding electrons)
Calculating the formal charge of every atom can be important to determine the most stable form of the molecule's Lewis structure. Generally, a Lewis structure with the lowest formal charge for all atoms will be the most stable one. In the case of PO(OH)₃, after the double bond is introduced, the formal charges minimize.
Step 7: Final Lewis Structure for PO(OH)₃
The final Lewis structure for PO(OH)₃ will show:
- A central phosphorus atom.
- Three hydroxyl groups (-OH) each single bonded to the phosphorus.
- One oxygen atom double bonded to the phosphorus.
- Lone pairs of electrons on the oxygen atoms to complete their octets.
Resonance Structures in PO(OH)₃
It's crucial to understand that the double bond between the phosphorus and one of the oxygen atoms is not static. The double bond can exist between phosphorus and any of the four oxygen atoms. This leads to resonance structures, where multiple Lewis structures can be drawn to represent the molecule. These resonance structures contribute to the overall structure of the molecule, and its properties.
Implications of the Lewis Structure of PO(OH)₃
The Lewis structure provides vital information about PO(OH)₃:
-
Molecular Geometry: The Lewis structure reveals the arrangement of atoms. This allows us to predict the molecular geometry using theories like VSEPR (Valence Shell Electron Pair Repulsion) theory. PO(OH)₃ exhibits a tetrahedral geometry around the phosphorus atom.
-
Polarity: The Lewis structure indicates the presence of polar bonds (due to the difference in electronegativity between P and O, and O and H). The overall molecule is polar due to the asymmetrical distribution of these polar bonds.
-
Acidity: The Lewis structure clearly shows the presence of three hydroxyl (-OH) groups. The bond between the oxygen and hydrogen in the hydroxyl group is polar. This is important to explain the molecule's acidic nature. The molecule can easily lose protons (H⁺), which explains its ability to act as a triprotic acid.
-
Reactivity: The Lewis structure helps predict how the molecule will react. The presence of the double bond, even as a resonance structure, and the polar nature make the molecule reactive.
Advanced Concepts and Applications
The Lewis structure of PO(OH)₃ serves as a foundation for understanding more complex concepts in chemistry, including:
-
Molecular Orbital Theory: This more sophisticated model provides a more detailed description of the bonding in PO(OH)₃.
-
Spectroscopy: The Lewis structure, along with other spectroscopic data, helps assign observed spectral features.
Conclusion: The Importance of Understanding Lewis Structures
The Lewis structure for PO(OH)₃, while seemingly simple, provides a powerful tool for understanding the molecule's structure, bonding, and reactivity. Mastering the construction and interpretation of Lewis structures is fundamental to success in chemistry. By carefully following the steps outlined, and by understanding the implications of the final structure, one can gain a deeper appreciation of the fundamental principles governing molecular interactions. This knowledge is crucial not only for academic purposes but also for various applications in fields like materials science and biochemistry. The ability to accurately represent and analyze molecular structures using tools like Lewis structures paves the way for further exploration and discovery in the broader realm of chemical understanding. Through a thorough understanding of resonance structures and formal charges, a comprehensive picture of the molecule's behavior can be constructed, which can then be applied for predicting its properties and reactivity.
Latest Posts
Latest Posts
-
When Using A Self Managed Team A Manager Should
Mar 17, 2025
-
Match Each Definition To The Level Of Protein Structure
Mar 17, 2025
-
A Fixed Position Production Layout Would Be Particularly Recommended If
Mar 17, 2025
-
When Direct Labor Costs Are Recorded
Mar 17, 2025
-
In Prompt Engineering Why It Is Important To Specify
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Lewis Structure For Po Oh 3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
