Lewis Dot Structure For No2 1
Holbox
Mar 31, 2025 · 6 min read
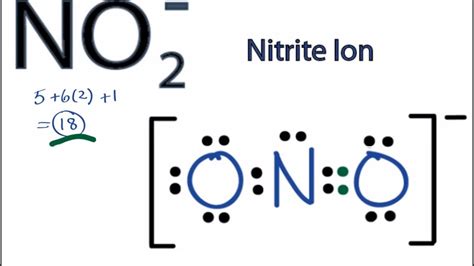
Table of Contents
- Lewis Dot Structure For No2 1
- Table of Contents
- Lewis Dot Structure for NO₂: A Comprehensive Guide
- Understanding the Basics: Valence Electrons and Octet Rule
- Constructing the Lewis Dot Structure for NO₂: Step-by-Step
- Resonance Structures of NO₂: Overcoming the Limitations
- Formal Charges and their Significance
- Molecular Geometry and Bond Angles of NO₂
- NO₂'s Polarity and Properties
- Applications and Importance of NO₂
- Conclusion: A Deeper Understanding of NO₂
- Latest Posts
- Latest Posts
- Related Post
Lewis Dot Structure for NO₂: A Comprehensive Guide
The Lewis dot structure, a fundamental concept in chemistry, provides a visual representation of the valence electrons in a molecule. Understanding this structure is crucial for predicting molecular geometry, polarity, and reactivity. This article delves deep into constructing and interpreting the Lewis dot structure for nitrogen dioxide (NO₂), a fascinating molecule with a unique structure and properties. We will explore multiple resonance structures, formal charges, and the implications for NO₂'s behavior.
Understanding the Basics: Valence Electrons and Octet Rule
Before we dive into the NO₂ Lewis structure, let's refresh some essential concepts. The Lewis dot structure is built upon the idea of valence electrons, which are the electrons in the outermost shell of an atom. These electrons participate in chemical bonding. The octet rule, a guiding principle in Lewis structure construction, states that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with eight valence electrons (like a noble gas). However, it's important to remember that the octet rule is a guideline, not an absolute law, and exceptions exist, especially for molecules containing elements beyond the second period.
Nitrogen (N) has five valence electrons (2s²2p³), and Oxygen (O) has six valence electrons (2s²2p⁴). To construct the Lewis dot structure for NO₂, we need to consider the total number of valence electrons available. With one nitrogen and two oxygens, we have a total of 5 + 6 + 6 = 17 valence electrons. This odd number immediately hints at the presence of an unpaired electron and potential radical behavior.
Constructing the Lewis Dot Structure for NO₂: Step-by-Step
-
Identify the Central Atom: Nitrogen, being less electronegative than oxygen, is the central atom.
-
Arrange the Atoms: Place the nitrogen atom in the center, and the two oxygen atoms surrounding it.
-
Connect Atoms with Single Bonds: Connect each oxygen atom to the nitrogen atom with a single bond. Each single bond represents two electrons. This uses four of our 17 valence electrons.
-
Distribute Remaining Electrons: We have 17 - 4 = 13 electrons left. Distribute these electrons to satisfy the octet rule for as many atoms as possible. Start by completing the octets of the oxygen atoms. Each oxygen atom needs six more electrons to complete its octet (remember, two are already involved in the single bond). This uses 12 electrons (6 for each oxygen).
-
Account for Unpaired Electrons: We have 13 - 12 = 1 electron remaining. This unpaired electron resides on the nitrogen atom, making NO₂ a radical.
This initial Lewis structure shows a nitrogen atom with only seven electrons and one oxygen with an incomplete octet. Therefore, this initial depiction is incomplete. We need to move on to a more accurate depiction involving resonance structures.
Resonance Structures of NO₂: Overcoming the Limitations
The initial Lewis structure we constructed doesn't accurately represent the bonding in NO₂. The molecule exhibits resonance, meaning there's more than one valid way to draw the Lewis structure. The actual structure is a hybrid of these resonance structures.
To account for this, we can form a double bond between nitrogen and one of the oxygen atoms. This redistributes electrons, providing a more stable structure that adheres better to the octet rule.
-
Resonance Structure 1: A double bond between the nitrogen atom and one oxygen atom, and a single bond between nitrogen and the other oxygen atom. This gives one oxygen atom a formal charge of -1, nitrogen a formal charge of +1, and the other oxygen atom a formal charge of 0.
-
Resonance Structure 2: A double bond between the nitrogen atom and the other oxygen atom, and a single bond between nitrogen and the first oxygen atom. This gives the first oxygen atom a formal charge of 0, nitrogen a formal charge of +1, and the second oxygen atom a formal charge of -1.
The actual molecule is a hybrid of these two resonance structures. The double bond is delocalized, meaning it's spread out over both nitrogen-oxygen bonds. This results in bond lengths that are intermediate between single and double bonds.
Formal Charges and their Significance
Formal charges help us assess the stability of different resonance structures. The formal charge of an atom is calculated as:
Formal charge = (Valence electrons) - (Non-bonding electrons) - (1/2 * Bonding electrons)
Calculating formal charges for each atom in the resonance structures clarifies the charge distribution. As mentioned above, the oxygen atoms will have formal charges of -1 and 0 while the nitrogen will have a formal charge of +1 in each structure, depending on which oxygen atom has the double bond. While neither of these structures is strictly more correct, the lower the sum of absolute values of the formal charges, the more stable the structure. This does not change whether the resonance structures are viable, it simply helps in understanding the distribution of electron density.
Molecular Geometry and Bond Angles of NO₂
The presence of resonance significantly impacts the molecular geometry and bond angles. NO₂ displays a bent molecular geometry due to the lone pair of electrons on the nitrogen atom. The bond angle is approximately 134°, slightly less than the ideal 120° predicted for a pure trigonal planar geometry. The repulsion from the lone pair causes the bond angle to compress.
NO₂'s Polarity and Properties
The presence of an unpaired electron and the polar nature of the N-O bonds makes NO₂ a polar molecule. The bent molecular geometry contributes to this polarity as the dipoles don't cancel out. This polarity significantly impacts its physical and chemical properties. For example, NO₂ is readily soluble in polar solvents. Its unpaired electron also accounts for its significant reactivity.
Applications and Importance of NO₂
Nitrogen dioxide is a crucial molecule in various chemical processes and is relevant in several different contexts:
-
Atmospheric Chemistry: NO₂ is a significant component of air pollution, contributing to acid rain and smog formation. It's formed through the combustion of fossil fuels and other industrial processes.
-
Industrial Applications: NO₂ has applications in the manufacture of nitric acid, a crucial industrial chemical used in the production of fertilizers and explosives.
-
Biological Significance: Although generally toxic, small amounts of nitrogen oxides are involved in biological processes like signaling pathways and regulation.
-
Material Science: NO₂ plays a role in certain material synthesis pathways and is studied for its potential role in various material modifications.
Conclusion: A Deeper Understanding of NO₂
The Lewis dot structure, while seemingly simple, provides a gateway to a deep understanding of molecular structure and behavior. The case of NO₂, with its resonance structures, formal charges, and odd number of valence electrons, beautifully illustrates the complexities and exceptions inherent in chemical bonding. The knowledge gained by constructing and interpreting the Lewis structure of NO₂ is essential for comprehending its role in atmospheric chemistry, industrial processes, and various other scientific fields. Through a comprehensive understanding of these fundamental concepts, we can better predict and explain the diverse properties and reactive nature of this important molecule. This detailed exploration provides a foundation for understanding more complex molecules and chemical interactions.
Latest Posts
Latest Posts
-
This Is A Tentative Explanation For A Natural Event
Apr 02, 2025
-
The Great Contribution Of Nicholas Copernicus Was To
Apr 02, 2025
-
Many Jurisdictions Organizations Configure Their Eocs Using The Standard
Apr 02, 2025
-
The Lungs Are Lateral To The Heart
Apr 02, 2025
-
Translate The Correct Sentences From Exercise 2 Into Your Language
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Lewis Dot Structure For No2 1 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
